Evaluation of the Mitral and Aortic Valves with Cardiac CT Angiography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Flow DHO8 7.8, Pg
Blood Flow DHO8 7.8, pg. 190 HS1/2017-2018 Circuits •Pulmonary circuit –The blood pathway between the right of the heart, to the lungs, and back to the left side of the heart. •Systemic circuit –The pathway between the left side of the heart, to the body, and back to the right side of the heart. The Pathway of Blood •Superior & Inferior Vena •Left Atrium Cava •Mitral Valve •Right Atrium •Left Ventricle •Tricuspid Valve •Aortic Semilunar Valve •Right Ventricle •Aorta •Pulmonary Semilunar -Arteries Valve -Arterioles •Pulmonary Artery -Capillaries •Lungs -Venules –Pulmonary Arterioles -Veins –Pulmonary Capillaries –Pulmonary Venules •Pulmonary Vein Blood Flow Through Heart Do You Know? • When blood leaves the left atrium, where does it go next? a) Aorta b) Left ventricle c) Right atrium d) Pulmonary artery And the answer is….A Do You Know? • After blood leaves the right atrium, what valve prevents the back flow? a) Pulmonary b) Mitral c) Tricuspid d) Aortic And the answer is…C Do You Know? • The right ventricle is the chamber of the heart that pumps blood for the pulmonary circulation. Based on this information, blood from the right ventricle is on its way to the _____. a) Liver b) Lungs c) Hands and feet And the answer is…B Do You Know? • Which of the following is correct order of blood flow for the right side of the heart? a) RA, Tricuspid valve, RV, PSLV, pulmonary artery b) RA, PSLV, RV, Tricuspid valve, pulmonary artery c) RA, Tricuspid valve, RV, pulmonary artery , PSLV And the answer is…A Do You Know? • Which of the following is correct order of blood flow for the left side of the heart? a) LA, Bicuspid valve, LV, ASLV, aorta b) LA, ASLV, LV, Bicuspid valve, aorta c) LA, Bicuspid valve, LV, ASLV, aorta And the answer is…C. -

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Cardiovascular Magnetic Resonance Pocket Guide
Series Editors Bernhard A. Herzog John P. Greenwood Sven Plein Cardiovascular Magnetic Resonance Congenital Heart Disease Pocket Guide Bernhard A. Herzog Ananth Kidambi George Ballard First Edition 2014 Congenital Pocket Guide Tetralogy / Foreword Pulmonary Atresia Standard Views TGA Difficult Scans Single Ventricle Sequential Ebstein Anomaly Segmental Analysis Coronary Artery Shunts Anomalies AV Disease / References Aortic Coarctation Terminology Series Editors Authors Bernhard A. Herzog Bernhard A. Herzog John P. Greenwood Ananth Kidambi Sven Plein George Ballard Foreword The role of cardiovascular magnetic resonance (CMR) in evaluating the adult population with congenital heart disease continues to expand. This pocket guide aims to provide a day-to-day companion for those new to congenital CMR and for those looking for a quick reference guide in routine practice. The booklet gives an overview of the most common abnormalities and interventions as well as post-operative complications. It also provides typical scan protocols, key issues and a guide for reporting for each topic. Bernhard A. Herzog Ananth Kidambi George Ballard The Cardiovascular Magnetic Resonance Pocket Guide represents the views of the ESC Working Group on Cardiovascular Magnetic Resonance and was arrived at after careful consideration of the available evidence at the time it was written. Health professionals are encouraged to take it fully into account when exercising their clinical judgment. This pocket guide does not, however, override the individual responsibility of health professionals to make appropriate decisions in the circumstances of the individual patients, in consultation with that patient and, where appropriate and necessary, the patient's guardian or carer. It is also the health professional's responsibility to verify the applicable rules and regulations applicable to drugs and devices at the time of prescription. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Having an Echocardiogram to Screen for a Bicuspid Aortic Valve
Having an echocardiogram to screen for a bicuspid aortic valve UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm What is a bicuspid aortic valve? The aortic valve sits between the main chamber of the heart (the left ventricle) and the aorta. Its function is to ensure that blood flows correctly forward from the left ventricle into the aorta. Normally it has three thin leaflets which open as the heart contracts and then close to prevent back-flow of blood towards the ventricle. Aortic valve Tricuspid aortic valve (normal) Bicuspid aortic valve (abnormal) Some people may be born with an aortic valve made up of only 2 leaflets. The valve is then called bicuspid. This may cause problems with the functioning of the valve in that it may be more prone to gradually becoming narrowed or leaky. Sometimes a bicuspid valve may be associated with widening of the portion of the aorta that is connected to it. Widening of the aorta may occur in some relatives even if they have an aortic valve with 3 leaflets. If this widening is significant it is known as an aortic aneurysm. Is a bicuspid valve a common problem? Studies have suggested that this is not an uncommon valve problem and may occur in up to 1 in 200 people. 2 | PI18_1443_03 Having an echocardiogram to screen for a bicuspid aortic valve Recently there has been evidence that this condition may be genetic and thus have a tendency to run in a family. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -

Heart Valve Disease: Mitral and Tricuspid Valves
Heart Valve Disease: Mitral and Tricuspid Valves Heart anatomy The heart has two sides, separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen- rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves that regulate blood flow. The upper chambers are called the left and right atria, and the lower chambers are called the left and right ventricles. The mitral valve is located on the left side of the heart, between the left atrium and the left ventricle. This valve has two leaflets that allow blood to flow from the lungs to the heart. The tricuspid valve is located on the right side of the heart, between the right atrium and the right ventricle. This valve has three leaflets and its function is to Cardiac Surgery-MATRIx Program -1- prevent blood from leaking back into the right atrium. What is heart valve disease? In heart valve disease, one or more of the valves in your heart does not open or close properly. Heart valve problems may include: • Regurgitation (also called insufficiency)- In this condition, the valve leaflets don't close properly, causing blood to leak backward in your heart. • Stenosis- In valve stenosis, your valve leaflets become thick or stiff, and do not open wide enough. This reduces blood flow through the valve. Blausen.com staff-Own work, CC BY 3.0 Mitral valve disease The most common problems affecting the mitral valve are the inability for the valve to completely open (stenosis) or close (regurgitation). -

Echo in Asymptomatic Mitral and Aortic Regurgitation
2017 ASE Florida | Orlando, FL October 9, 2017 | 10:40 – 11:00 PM | 20 min | Grand Harbor Ballroom South Echo in Asymptomatic Mitral and Aortic Regurgitation Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology | Echo Lab Associate Professor of Medicine Disclosures Speakers Bureau (Philips, Medtronic) Advisory Board (Siemens) Regurgitation Axioms ▪Typically, regurgitation is NOT symptomatic unless severe ▪The opposite is not true: Severe regurgitation may be asymptomatic ▪ Chronic regurgitation leads to chamber dilatation on either side of the regurgitant valve Regurgitation Discovery ▪ Regurgitation as a anatomic entity was recognized in the 17th century ▪ Regurgitation was first clinically diagnosed by auscultation in the 19th century, well before the advent of echocardiography First Use of Regurgitation Term in English 1683 W. Charleton Three Anat. Lect. i. 18 Those [valves] that are placed in the inlet and outlet of the left Ventricle, to obviate the regurgitation of the bloud into the arteria venosa, and out of the aorta into the left Ventricle. Walter Charleton (1619 – 1707) English Physician Heart Murmur OXFORD ENGLISH DICTIONARY DEFINITION ▪ Any of various auscultatory sounds ▪ Adventitious sounds of cardiac or vascular origin [that is, separate from standard heart sounds: S1, S2, S3, S4] ▪ Sometimes of no significance ▪ But sometimes caused by valvular lesions of the heart or other diseases of the Στῆθος : Stēthos = chest circulatory system René Laënnec Stethoscope (1781 – 1826) (‘Chest examiner’) French Physician Hollow wooden cylinder Inventor of stethoscope in 1816 Laënnec Performing Auscultation Painted by Robert Alan Thom (1915 – 1979), American illustrator Commissioned by Parke, Davis & Co. 1816 1832 René Laënnec, James Hope French physician British physician Invents MONAURAL stethoscope separates MS from MR murmur 1852 1862 George Cammann Austin Flint Sr. -
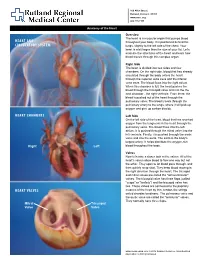
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities. -

Congenital Mitral Incompetence and Coarctation Ofaorta
Thorax: first published as 10.1136/thx.27.6.729 on 1 November 1972. Downloaded from Thorax (1972), 27, 729. Congenital mitral incompetence and coarctation of aorta Report of two cases treated surgically' ABDEL K. TERZAKI2, ROBERT D. LEACHMAN3, M. KHALIL ALI, GRADY L. HALLMAN, and DENTON A. COOLEY Department of Medicine and the Cora and Webb Mading Department of Surgery, Baylor University College of Medicine, and the Cardiology and Surgical Sections of the Texas Heart Institute of St. Luke's Episcopal and Texas Children's Hospitals, Houston, Texas, U.S.A. Two patients with congenital mitral incompetence and coarctation of the aorta are presented. One patient had associated patent ductus arteriosus, bicuspid aortic valve, and endocardial fibro- elastosis. The diagnosis in the two patients presented is well established by clinical, laboratory, and surgical findings and also by necropsy examination in one case. It is proposed that the rarity of reported cases in the literature may have resulted from the frequent diagnosis of left ventricular failure in infancy secondary to coarctation, leading to the assumption that a mitral insufficiency murmur, when present, is due to functional regurgitation. Likewise, the murmur may be mistakenly thought to originate from a ventricular septal defect. The diagnosis of coarctation of the aorta presented no problem in either patient, while detection of the mitral incompetence was difficult. Coarctation of the aorta complicated by copyright. pulmonary hypertension in the absence of intracardiac shunt should draw attention to the possi- bility of associated mitral incompetence. Congestive heart failure, especially after correction of coarctation, was also an indication of possible associated mitral insufficiency.