Symptomatic Presentations of Severe Aortic Stenosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -
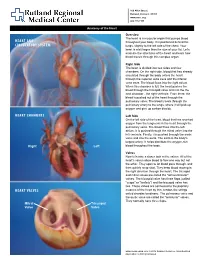
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities. -

Transcatheter Aortic Valve Replacement
What is TAVR? Cardiac Catheterization: Important things to know that will help you get ready Transcatheter Aortic Valve Replacement (TAVR) is a procedure Your doctor will tell if you need to stop eating or drinking to fix the aortic valve without taking out the old valve. A TAVR before your procedure. Your doctor also will tell you if you does not need open heart surgery and the heart does not need must stop taking any medications before the procedure. to be stopped. Catheterization Lab In the Pre-Operative (Pre-Op) Room before your The surgeon puts a catheter (thin tube) into an artery in your Cardiac Catheterization upper leg or through a small cut in your chest. The catheter will • You will wear a hospital gown. We will ask you to take off all Transcatheter Aortic Valve carry a new valve to your heart. your clothing (even underwear), jewelry, dentures, glasses, Replacement (TAVR) hearing aids, etc. • An intravenous line (IV) may be put into a vein in your arm • We will prepare and clean the catheter site (where the catheter goes into your body). We will clean your skin with a special wash that kills germs. We may need to trim body hair. • We will ask you to empty your bladder (pee) before your procedure After Your Cardiac Catheterization • You may be on bed rest (lying flat) for 2 to 6 hours. To lower the risk of bleeding, we do not want you to bend your body at the catheter site (where the catheter went into your body) • Your nurse will often check your vital signs (blood pressure, heart rate, temperature) and catheter site • You must use a urinal or bed pan until you can safely stand and walk to the bathroom • While you are healing, do not do strenuous exercise (such as running or lifting weights). -
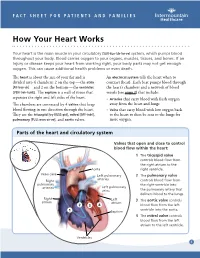
How Your Heart Works
FACT SHEET FOR PATIENTS AND FAMILIES How Your Heart Works Your heart is the main muscle in your circulatory [SUR-kue-luh-tor-ee] system, which pumps blood throughout your body. Blood carries oxygen to your organs, muscles, tissues, and bones. If an injury or disease keeps your heart from working right, your body parts may not get enough oxygen. This can cause additional health problems or even death. The heart is about the size of your fist and is An electrical system tells the heart when to divided into 4 chambers: 2 on the top — the atria contract (beat). Each beat pumps blood through [AY-tree-uh] — and 2 on the bottom — the ventricles the heart’s chambers and a network of blood [VEN-treh-kuhlz]. The septum is a wall of tissue that vessels (see page 2) that include: separates the right and left sides of the heart. • Arteries that carry blood with fresh oxygen The chambers are connected by 4 valves that keep away from the heart and lungs blood flowing in one direction through the heart. • Veins that carry blood with low oxygen back They are the tricuspid [try-KUSS-pid], mitral [MY-truhl], to the heart to then be sent to the lungs for pulmonary [PULL-mon-air-ee], and aortic valves. more oxygen. Parts of the heart and circulatory system Valves that open and close to control blood flow within the heart: 1 The tricuspid valve controls blood flow from the right atrium to the Aorta right ventricle. Vena cava Left pulmonary 2 The pulmonary valve arteries Right controls blood flow from pulmonary the right ventricle into arteries Left pulmonary veins the pulmonary artery that delivers blood to the lungs. -

Bicuspid Aortic Valve
© 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Bicuspid Aortic Valve A bicuspid aortic valve is form of congenital heart disease where the aortic valve only has two leaflets, instead of three. This typically results from fusion (joining) of two cusps of the valve along their coaptation point. A bicuspid aortic valve occurs in 1-2% of the population. It can occur alone or be associated with other left-sided heart lesions, such as mitral valve abnormalities (see mitral stenosis), or coarctation of the aorta. Symptoms and presentation can vary for patients with bicuspid aortic valve depending upon the degree of stenosis (narrowing) or regurgitation (leaking) of the valve. Physical Exam/Symptoms: Most patients with bicuspid aortic valve have no symptoms (asymptomatic), unless there is associated aortic stenosis (AS) (narrowing) or regurgitation (leaking) (AR) Most children are asymptomatic with mild to moderate AS. Fatigue, chest pain with exertion, or syncope (fainting) may occur in severe AS. In critical AS, neonates develop poor perfusion, pulmonary edema (fluid retention in the lungs) within days or weeks after birth as the ductus arteriosus (see Patent Ductus Arteriosus) closes. Clinical picture may resemble that of sepsis (severe infection). Murmur of AS: Harsh, grade II/VI systolic murmur heard best at the second left intercostal space, with transmission to the head and neck. -

Endocardial Thickening Associated with Diseased Valves by W
Br Heart J: first published as 10.1136/hrt.23.2.155 on 1 March 1961. Downloaded from ENDOCARDIAL THICKENING ASSOCIATED WITH DISEASED VALVES BY W. J. S. STILL From the Royal Free Hospital Received September 16, 1960 Focal endocardial thickening is often found in relation to diseased valves and is particularly frequent on the upper part of the interventricular septum in association with aortic regurgitation. Some of the endocardial thickenings may be in the form of endocardial pockets or false valves (Fig. 1), and at times a similar type oflocalized thickening is found in the aortic intimajust above the http://heart.bmj.com/ on September 25, 2021 by guest. Protected copyright. FIG. Endocardial pocket formation associated with aortic regurgi tation and stenosis. A traumatic intimal thickening is present on the aorta just above the valve valve. It is now generally accepted that these lesions are the result of the abnormal jets and eddies of blood set up by the distorted valve impinging on these particular areas of the endocardium or intima (Krasso, 1929; Saphir, 1930; Ingham and Henthorne, 1938; Hellerstein, 1947). 155 Br Heart J: first published as 10.1136/hrt.23.2.155 on 1 March 1961. Downloaded from 156 W. J. S. STILL What appears to be a related type of endocardial thickening constitutes one form of sub-aortic stenosis. This consists of a semi-circular or annular bar of endocardium situated just below an aortic valve, which is usually stenosed or otherwise diseased. Gruenwald (1947) considered this type of endocardial thickening to be the result of inflammation, but it seems likely that many of the examples of subaortic stenosis combined with aortic valve disease are due, like the focal thickenings, to abnormal blood flow (Saphir, 1953). -

AORTIC VALVE REPLACEMENT with PERCEVAL SUTURELESS VALVE Patient’S Guide
AORTIC VALVE REPLACEMENT WITH PERCEVAL SUTURELESS VALVE Patient’s guide Perceval - Patient’s guide 1 TABLE OF CONTENTS Scope ................................................................................................................................................................. 3 The Human Heart .............................................................................................................................................. 4 How Does The Heart Work? .............................................................................................................................. 5 Heart Valve Diseases ......................................................................................................................................... 5 Valve Stenosis ................................................................................................................................................ 6 Aortic Regurgitation ...................................................................................................................................... 6 What Are the Symptoms Of Aortic Valve Disease? ........................................................................................... 7 What Are the Options in Heart Valve Replacement? ........................................................................................ 7 Mechanical Valves ......................................................................................................................................... 8 Tissue Valves ................................................................................................................................................. -

Anatomy of the True Interatrial Septum for Transseptal Access to the Left Atrium
Annals of Anatomy 205 (2016) 60–64 Contents lists available at ScienceDirect Annals of Anatomy jou rnal homepage: www.elsevier.com/locate/aanat Research article Anatomy of the true interatrial septum for transseptal access to the left atrium 1 ∗,1 Wiesława Klimek-Piotrowska , Mateusz K. Hołda , Mateusz Koziej, Katarzyna Piatek,˛ Jakub Hołda Department of Anatomy, Jagiellonian University Medical College, Cracow, Poland a r t i c l e i n f o a b s t r a c t Article history: Clinical anatomy of the interatrial septum is treacherous, difficult and its unfamiliarity can cause many Received 6 January 2016 serious complications. This work aims to create an anatomical map of the true interatrial septum. An Received in revised form 23 January 2016 appreciation of the anatomical situation is essential for safe and efficacious transseptal access from the Accepted 25 January 2016 right atrium to the left heart chambers. Examination of 135 autopsied human hearts (Caucasian) of 2 both sexes (28% females) aged from 19 to 94 years old (47.0 ± 18.2) with BMI = 27.1 ± 6.0 kg/m was Keywords: conducted. Focus was specifically targeted on the assessment of the fossa ovalis, patent foramen ovale Transseptal puncture (PFO), and right-sided septal pouch (RSP) morphology. Mean values of cranio-caudal and antero-posterior Septal pouch fossa ovalis diameters were 12.1 ± 3.6 and 14.1 ± 3.6 mm, respectively. The fossa ovalis was situated an Patent foramen ovale average of 10.1 ± 4.4 mm above the inferior vena cava ostium, 20.7 ± 5.2 mm from the right atrioventric- Fossa ovalis ular ring, and 12.6 ± 5.2 mm under the right atrium roof. -

Heart Valve Disease
Treatment Guide Heart Valve Disease Heart valve disease refers to any of several condi- TABLE OF CONTENTS tions that prevent one or more of the valves in the What causes valve disease? .................................. 2 heart from functioning adequately to assure prop- er circulation. Left untreated, heart valve disease What are the symptoms of heart valve disease? ....... 5 can reduce quality of life and become life-threat- How is valve disease diagnosed? ............................ 6 ening. In many cases, heart valves can be surgi- What treatments are available? .............................. 8 cally repaired or replaced, restoring normal func- What are the types of valve surgery? ...................... 9 tion and allowing a return to normal activities. What can I expect before and after surgery? .......... 13 Cleveland Clinic’s Sydell and Arnold Miller How can I protect my heart valves? ...................... 17 Family Heart & Vascular Institute is one of the largest centers in the country for the diagnosis and treatment of heart valve disease. The decision to prescribe medical treatment or proceed with USING THIS GUIDE surgical repair or replacement is based on the Please use this guide as a resource as you examine your type of heart valve disease you have, the severity treatment options. Remember, it is every patient’s right of damage, your age and your medical history. to ask questions, and to seek a second opinion. To make an appointment with a heart valve specialist at Cleveland Clinic, call 216.444.6697. CLEVELAND CLINIC | HEART VALVE DISEASE TREATMENT GUIDE About Valve Disease The heart valves How the Valves Work Heart valve disease means one of the heart valves isn’t working properly because The heart has four valves — one for of valvular stenosis (narrowing of the valves) or valvular insufficiency (“leaky” valve). -

Anatomy and Physiology of the Cardiovascular System
Chapter © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 5 NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Anatomy© Jonesand & Physiology Bartlett Learning, LLC of © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION the Cardiovascular System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION OUTLINE Aortic arch: The second section of the aorta; it branches into Introduction the brachiocephalic trunk, left common carotid artery, and The Heart left subclavian artery. Structures of the Heart Aortic valve: Located at the base of the aorta, the aortic Conduction System© Jones & Bartlett Learning, LLCvalve has three cusps and opens© Jonesto allow blood & Bartlett to leave the Learning, LLC Functions of the HeartNOT FOR SALE OR DISTRIBUTIONleft ventricle during contraction.NOT FOR SALE OR DISTRIBUTION The Blood Vessels and Circulation Arteries: Elastic vessels able to carry blood away from the Blood Vessels heart under high pressure. Blood Pressure Arterioles: Subdivisions of arteries; they are thinner and have Blood Circulation muscles that are innervated by the sympathetic nervous Summary© Jones & Bartlett Learning, LLC system. © Jones & Bartlett Learning, LLC Atria: The upper chambers of the heart; they receive blood CriticalNOT Thinking FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Websites returning to the heart. Review Questions Atrioventricular node (AV node): A mass of specialized tissue located in the inferior interatrial septum beneath OBJECTIVES the endocardium; it provides the only normal conduction pathway between the atrial and ventricular syncytia.