Multimodality Imaging of the Anatomy of the Aortic Root
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ventricles
Guest Editorial Evolution of the Ventricles Solomon Victor, FRCS, FRCP We studied the evolution of ventricles by macroscopic examination of the hearts of Vijaya M. Nayak, MS marine cartilaginous and bony fish, and by angiocardiography and gross examination of Raveen Rajasingh, MPhil the hearts of air-breathing freshwater fish, frogs, turtles, snakes, and crocodiles. A right-sided, thin-walled ventricular lumen is seen in the fish, frog, turtle, and snake. In fish, there is external symmetry of the ventricle, internal asymmetry, and a thick- walled left ventricle with a small inlet chamber. In animals such as frogs, turtles, and snakes, the left ventricle exists as a small-cavitied contractile sponge. The high pressure generated by this spongy left ventricle, the direction of the jet, the ventriculoarterial ori- entation, and the bulbar spiral valve in the frog help to separate the systemic and pul- monary circulations. In the crocodile, the right aorta is connected to the left ventricle, and there is a complete interventricular septum and an improved left ventricular lumen when compared with turtles and snakes. The heart is housed in a rigid pericardial cavity in the shark, possibly to protect it from changing underwater pressure. The pericardial cavity in various species permits move- ments of the heart-which vary depending on the ventriculoarterial orientation and need for the ventricle to generate torque or spin on the ejected blood- that favor run-off into the appropriate arteries and their branches. In the lower species, it is not clear whether the spongy myocardium contributes to myocardial oxygenation. In human beings, spongy myocardium constitutes a rare form of congenital heart disease. -

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -

PERCEVAL SUTURELESS AORTIC HEART VALVE Instructions for Use
HVV_LS-850-0002 Rev X03 PERCEVAL SUTURELESS AORTIC HEART VALVE Instructions for Use CAUTION: Federal Law (USA) restricts the device to sale by or on the order of a physician. SYMBOLS CAUTION: SEE MANUAL FOR INSTRUCTIONS/WARNINGS CONTENTS STERILIZED USING ASEPTIC PROCESSING TECHNIQUE USE BY STORE BETWEEN 5°C AND 25°C SINGLE USE ONLY DO NOT RESTERILIZE CATALOGUE NUMBER SERIAL NUMBER SIZE MANUFACTURER QUANTITY INCLUDED IN PACKAGE DO NOT USE IF PACKAGE IS DAMAGED THIS WAY UP MR CONDITIONAL 1. DESCRIPTION Perceval is a bioprosthetic valve designed to replace a diseased native or a malfunctioning prosthetic aortic valve via open heart surgery, with the unique characteristic of allowing sutureless positioning and anchoring at the implant site. The choice of materials and configuration ensures the device biocompatibility and hemocompatibility. The Perceval prosthesis consists of a tissue component made from bovine pericardium and a self-expandable Nitinol stent, which has the dual role of supporting the valve and fixing it in place. Perceval tissue heart valve is supplied unmounted. Prior to implantation the prosthesis diameter is reduced to a suitable size for loading it on the holder. The valve is then positioned and released in the aortic root, where the stent design and its ability to apply a radial force to the annulus allow stable anchoring of the device. 2. AVAILABLE MODELS The Perceval aortic model is available in four sizes: size S, size M, size L, and size XL. The prosthesis height is 31.0, 33.0, 35.5, and 37.5 mm, respectively. Each size is suitable for a range of aortic annuli and sinotubular junction (STJ) diameters. -

Cardiovascular System ANS 215 Physiology and Anatomy of Domesticated Animals
Cardiovascular System ANS 215 Physiology and Anatomy of Domesticated Animals I. Structure and Function A. Heart is a cone-shaped, hollow, muscular structure located in the thorax. B. Larger arteries and veins are continuous with the heart as its base. 1. Base is directed upward (dorsal) and forward (cranial). 2. Opposite end of the cone is known as the apex C. Membrane around the heart is known as the pericardium 1. Membrane next to hear fuses with the heart muscle and is called the visceral pericardium or epicardium 2. outer membrane is parietal pericardium 3. apex is free 4. Inflammation of the pericardium is called pericarditis. a. increase in fluid in pericardium b. traumatic pericarditis (hardware) disease in cattle 1 Left view of bovine thorax and abdomen showing location of the heart relative to the stomach. Foreign objects (nails, wire), sometimes ingested by cattle, accumulate in the reticulum ( one of the bovine forestomachs). Contraction of the reticulum can force pointed objects through the reticulum wall and the diaphragm, causing final penetration of the pericardium and subsequent inflammation (pericarditis). 2 D. Myocardium 1. Muscular part of the heart which forms the walls for the chambers 2. Heart chambers (4) divided into left and right side of the heart a. Each side has an atrium and ventricle. b. Each atrium has an extension known as the auricle. c. Atria receive blood from veins and ventricles receive blood from atria. Computer image of a cross sectional view of the heart at the ventricular level showing the chordae tendinae and the relative thickness of the myocardium. -

Growth and Remodeling of Atrioventricular Heart Valves: a Potential Target for Pharmacological Treatment? Manuel K
Available online at www.sciencedirect.com Current Opinion in ScienceDirect Biomedical Engineering Growth and remodeling of atrioventricular heart valves: A potential target for pharmacological treatment? Manuel K. Rausch Abstract backflow or regurgitation of blood. These vital functions Atrioventricular heart valves, that is, the mitral valve and the depend on a well-orchestrated interplay between the tricuspid valve, play vital roles in our cardiovascular system. valves’ components, that is, the valve leaflets, the valve Disease of these valves is, therefore, a significant source of annulus, the chordae tendineae, and the papillary morbidity and mortality. Unfortunately, current treatment op- muscles, refer Figure 1a. In this role, their central tions are suboptimal with significant rates of failure. It was only components, the valve leaflets, are exposed to hemo- recently that we have begun to appreciate that the atrioven- dynamic shear stresses, radial tensile forces at the tricular heart valve leaflets are not just passive flaps, but chordal insertion sites, circumferential tensile forces at actively (mal)adapting tissues. This discovery sheds new light their annular insertion, biaxial stretch due to the on disease mechanisms and provides, thus, possible path- transvalvular pressure, and compressive forces in the ways to new treatments. In this current opinion piece, we coaptation zone. This complex loading regime is cycli- examine the state of our knowledge about the (mal)adaptive cally repeated with every heartbeat for billions of times mechanisms (physiological and pathological growth and throughout our lifetime [1,2]. Ostensibly, these loading remodeling) of the atrioventricular heart valves. Furthermore, modes determine the valves’ microstructure and we review the evidence that suggests that valve maladaptation consequently their mechanical properties [3]. -

Readingsample
Integrating Cardiology for Nuclear Medicine Physicians A Guide to Nuclear Medicine Physicians Bearbeitet von Assad Movahed, Gopinath Gnanasegaran, John Buscombe, Margaret Hall 1. Auflage 2008. Buch. xiv, 544 S. Hardcover ISBN 978 3 540 78673 3 Format (B x L): 21 x 27,9 cm Weitere Fachgebiete > Medizin > Sonstige Medizinische Fachgebiete > Nuklearmedizin, Radiotherapie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Chapter The Heart: Anatomy, Physiology and 1 Exercise Physiology Syed Shah, Gopinath Gnanasegaran, Jeanette Sundberg-Cohon, and John R Buscombe Contents 1.1 Introduction 1.1 Introduction . 3 1.2 Anatomy of the Heart . 3 The impact of anatomy on medicine was first recognised 1.2.1 Chamber and Valves . 4 by Andreas Vesalius during the 16th century [1] and 1.2.2 Cardiac Cell and Cardiac Muscle ......... 4 from birth to death, the heart is the most talked about 1.2.3 Coronary Arteries and Cardiac Veins . 6 organ of the human body. It is the centre of attraction 1.2.4 Venous Circulation ..................... 6 for people from many lifestyles, such as philosophers, 1.2.5 Nerve Supply of the Heart ............... 9 artists, poets and physicians/surgeons. The heart is one 1.2.6 Conduction System of the Heart . 10 of the most efficient organs in the human body and 1.3 Physiology of the Heart . -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Heart Valve Disease: Mitral and Tricuspid Valves
Heart Valve Disease: Mitral and Tricuspid Valves Heart anatomy The heart has two sides, separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen- rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves that regulate blood flow. The upper chambers are called the left and right atria, and the lower chambers are called the left and right ventricles. The mitral valve is located on the left side of the heart, between the left atrium and the left ventricle. This valve has two leaflets that allow blood to flow from the lungs to the heart. The tricuspid valve is located on the right side of the heart, between the right atrium and the right ventricle. This valve has three leaflets and its function is to Cardiac Surgery-MATRIx Program -1- prevent blood from leaking back into the right atrium. What is heart valve disease? In heart valve disease, one or more of the valves in your heart does not open or close properly. Heart valve problems may include: • Regurgitation (also called insufficiency)- In this condition, the valve leaflets don't close properly, causing blood to leak backward in your heart. • Stenosis- In valve stenosis, your valve leaflets become thick or stiff, and do not open wide enough. This reduces blood flow through the valve. Blausen.com staff-Own work, CC BY 3.0 Mitral valve disease The most common problems affecting the mitral valve are the inability for the valve to completely open (stenosis) or close (regurgitation). -
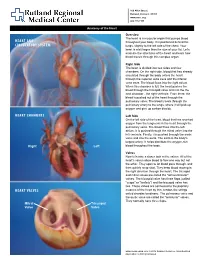
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities. -

Transcatheter Aortic Valve Replacement
What is TAVR? Cardiac Catheterization: Important things to know that will help you get ready Transcatheter Aortic Valve Replacement (TAVR) is a procedure Your doctor will tell if you need to stop eating or drinking to fix the aortic valve without taking out the old valve. A TAVR before your procedure. Your doctor also will tell you if you does not need open heart surgery and the heart does not need must stop taking any medications before the procedure. to be stopped. Catheterization Lab In the Pre-Operative (Pre-Op) Room before your The surgeon puts a catheter (thin tube) into an artery in your Cardiac Catheterization upper leg or through a small cut in your chest. The catheter will • You will wear a hospital gown. We will ask you to take off all Transcatheter Aortic Valve carry a new valve to your heart. your clothing (even underwear), jewelry, dentures, glasses, Replacement (TAVR) hearing aids, etc. • An intravenous line (IV) may be put into a vein in your arm • We will prepare and clean the catheter site (where the catheter goes into your body). We will clean your skin with a special wash that kills germs. We may need to trim body hair. • We will ask you to empty your bladder (pee) before your procedure After Your Cardiac Catheterization • You may be on bed rest (lying flat) for 2 to 6 hours. To lower the risk of bleeding, we do not want you to bend your body at the catheter site (where the catheter went into your body) • Your nurse will often check your vital signs (blood pressure, heart rate, temperature) and catheter site • You must use a urinal or bed pan until you can safely stand and walk to the bathroom • While you are healing, do not do strenuous exercise (such as running or lifting weights). -
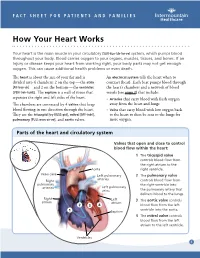
How Your Heart Works
FACT SHEET FOR PATIENTS AND FAMILIES How Your Heart Works Your heart is the main muscle in your circulatory [SUR-kue-luh-tor-ee] system, which pumps blood throughout your body. Blood carries oxygen to your organs, muscles, tissues, and bones. If an injury or disease keeps your heart from working right, your body parts may not get enough oxygen. This can cause additional health problems or even death. The heart is about the size of your fist and is An electrical system tells the heart when to divided into 4 chambers: 2 on the top — the atria contract (beat). Each beat pumps blood through [AY-tree-uh] — and 2 on the bottom — the ventricles the heart’s chambers and a network of blood [VEN-treh-kuhlz]. The septum is a wall of tissue that vessels (see page 2) that include: separates the right and left sides of the heart. • Arteries that carry blood with fresh oxygen The chambers are connected by 4 valves that keep away from the heart and lungs blood flowing in one direction through the heart. • Veins that carry blood with low oxygen back They are the tricuspid [try-KUSS-pid], mitral [MY-truhl], to the heart to then be sent to the lungs for pulmonary [PULL-mon-air-ee], and aortic valves. more oxygen. Parts of the heart and circulatory system Valves that open and close to control blood flow within the heart: 1 The tricuspid valve controls blood flow from the right atrium to the Aorta right ventricle. Vena cava Left pulmonary 2 The pulmonary valve arteries Right controls blood flow from pulmonary the right ventricle into arteries Left pulmonary veins the pulmonary artery that delivers blood to the lungs.