Heart – Inflammation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ventricles
Guest Editorial Evolution of the Ventricles Solomon Victor, FRCS, FRCP We studied the evolution of ventricles by macroscopic examination of the hearts of Vijaya M. Nayak, MS marine cartilaginous and bony fish, and by angiocardiography and gross examination of Raveen Rajasingh, MPhil the hearts of air-breathing freshwater fish, frogs, turtles, snakes, and crocodiles. A right-sided, thin-walled ventricular lumen is seen in the fish, frog, turtle, and snake. In fish, there is external symmetry of the ventricle, internal asymmetry, and a thick- walled left ventricle with a small inlet chamber. In animals such as frogs, turtles, and snakes, the left ventricle exists as a small-cavitied contractile sponge. The high pressure generated by this spongy left ventricle, the direction of the jet, the ventriculoarterial ori- entation, and the bulbar spiral valve in the frog help to separate the systemic and pul- monary circulations. In the crocodile, the right aorta is connected to the left ventricle, and there is a complete interventricular septum and an improved left ventricular lumen when compared with turtles and snakes. The heart is housed in a rigid pericardial cavity in the shark, possibly to protect it from changing underwater pressure. The pericardial cavity in various species permits move- ments of the heart-which vary depending on the ventriculoarterial orientation and need for the ventricle to generate torque or spin on the ejected blood- that favor run-off into the appropriate arteries and their branches. In the lower species, it is not clear whether the spongy myocardium contributes to myocardial oxygenation. In human beings, spongy myocardium constitutes a rare form of congenital heart disease. -

Blood Flow DHO8 7.8, Pg
Blood Flow DHO8 7.8, pg. 190 HS1/2017-2018 Circuits •Pulmonary circuit –The blood pathway between the right of the heart, to the lungs, and back to the left side of the heart. •Systemic circuit –The pathway between the left side of the heart, to the body, and back to the right side of the heart. The Pathway of Blood •Superior & Inferior Vena •Left Atrium Cava •Mitral Valve •Right Atrium •Left Ventricle •Tricuspid Valve •Aortic Semilunar Valve •Right Ventricle •Aorta •Pulmonary Semilunar -Arteries Valve -Arterioles •Pulmonary Artery -Capillaries •Lungs -Venules –Pulmonary Arterioles -Veins –Pulmonary Capillaries –Pulmonary Venules •Pulmonary Vein Blood Flow Through Heart Do You Know? • When blood leaves the left atrium, where does it go next? a) Aorta b) Left ventricle c) Right atrium d) Pulmonary artery And the answer is….A Do You Know? • After blood leaves the right atrium, what valve prevents the back flow? a) Pulmonary b) Mitral c) Tricuspid d) Aortic And the answer is…C Do You Know? • The right ventricle is the chamber of the heart that pumps blood for the pulmonary circulation. Based on this information, blood from the right ventricle is on its way to the _____. a) Liver b) Lungs c) Hands and feet And the answer is…B Do You Know? • Which of the following is correct order of blood flow for the right side of the heart? a) RA, Tricuspid valve, RV, PSLV, pulmonary artery b) RA, PSLV, RV, Tricuspid valve, pulmonary artery c) RA, Tricuspid valve, RV, pulmonary artery , PSLV And the answer is…A Do You Know? • Which of the following is correct order of blood flow for the left side of the heart? a) LA, Bicuspid valve, LV, ASLV, aorta b) LA, ASLV, LV, Bicuspid valve, aorta c) LA, Bicuspid valve, LV, ASLV, aorta And the answer is…C. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

PERCEVAL SUTURELESS AORTIC HEART VALVE Instructions for Use
HVV_LS-850-0002 Rev X03 PERCEVAL SUTURELESS AORTIC HEART VALVE Instructions for Use CAUTION: Federal Law (USA) restricts the device to sale by or on the order of a physician. SYMBOLS CAUTION: SEE MANUAL FOR INSTRUCTIONS/WARNINGS CONTENTS STERILIZED USING ASEPTIC PROCESSING TECHNIQUE USE BY STORE BETWEEN 5°C AND 25°C SINGLE USE ONLY DO NOT RESTERILIZE CATALOGUE NUMBER SERIAL NUMBER SIZE MANUFACTURER QUANTITY INCLUDED IN PACKAGE DO NOT USE IF PACKAGE IS DAMAGED THIS WAY UP MR CONDITIONAL 1. DESCRIPTION Perceval is a bioprosthetic valve designed to replace a diseased native or a malfunctioning prosthetic aortic valve via open heart surgery, with the unique characteristic of allowing sutureless positioning and anchoring at the implant site. The choice of materials and configuration ensures the device biocompatibility and hemocompatibility. The Perceval prosthesis consists of a tissue component made from bovine pericardium and a self-expandable Nitinol stent, which has the dual role of supporting the valve and fixing it in place. Perceval tissue heart valve is supplied unmounted. Prior to implantation the prosthesis diameter is reduced to a suitable size for loading it on the holder. The valve is then positioned and released in the aortic root, where the stent design and its ability to apply a radial force to the annulus allow stable anchoring of the device. 2. AVAILABLE MODELS The Perceval aortic model is available in four sizes: size S, size M, size L, and size XL. The prosthesis height is 31.0, 33.0, 35.5, and 37.5 mm, respectively. Each size is suitable for a range of aortic annuli and sinotubular junction (STJ) diameters. -

Cardiovascular System ANS 215 Physiology and Anatomy of Domesticated Animals
Cardiovascular System ANS 215 Physiology and Anatomy of Domesticated Animals I. Structure and Function A. Heart is a cone-shaped, hollow, muscular structure located in the thorax. B. Larger arteries and veins are continuous with the heart as its base. 1. Base is directed upward (dorsal) and forward (cranial). 2. Opposite end of the cone is known as the apex C. Membrane around the heart is known as the pericardium 1. Membrane next to hear fuses with the heart muscle and is called the visceral pericardium or epicardium 2. outer membrane is parietal pericardium 3. apex is free 4. Inflammation of the pericardium is called pericarditis. a. increase in fluid in pericardium b. traumatic pericarditis (hardware) disease in cattle 1 Left view of bovine thorax and abdomen showing location of the heart relative to the stomach. Foreign objects (nails, wire), sometimes ingested by cattle, accumulate in the reticulum ( one of the bovine forestomachs). Contraction of the reticulum can force pointed objects through the reticulum wall and the diaphragm, causing final penetration of the pericardium and subsequent inflammation (pericarditis). 2 D. Myocardium 1. Muscular part of the heart which forms the walls for the chambers 2. Heart chambers (4) divided into left and right side of the heart a. Each side has an atrium and ventricle. b. Each atrium has an extension known as the auricle. c. Atria receive blood from veins and ventricles receive blood from atria. Computer image of a cross sectional view of the heart at the ventricular level showing the chordae tendinae and the relative thickness of the myocardium. -

Growth and Remodeling of Atrioventricular Heart Valves: a Potential Target for Pharmacological Treatment? Manuel K
Available online at www.sciencedirect.com Current Opinion in ScienceDirect Biomedical Engineering Growth and remodeling of atrioventricular heart valves: A potential target for pharmacological treatment? Manuel K. Rausch Abstract backflow or regurgitation of blood. These vital functions Atrioventricular heart valves, that is, the mitral valve and the depend on a well-orchestrated interplay between the tricuspid valve, play vital roles in our cardiovascular system. valves’ components, that is, the valve leaflets, the valve Disease of these valves is, therefore, a significant source of annulus, the chordae tendineae, and the papillary morbidity and mortality. Unfortunately, current treatment op- muscles, refer Figure 1a. In this role, their central tions are suboptimal with significant rates of failure. It was only components, the valve leaflets, are exposed to hemo- recently that we have begun to appreciate that the atrioven- dynamic shear stresses, radial tensile forces at the tricular heart valve leaflets are not just passive flaps, but chordal insertion sites, circumferential tensile forces at actively (mal)adapting tissues. This discovery sheds new light their annular insertion, biaxial stretch due to the on disease mechanisms and provides, thus, possible path- transvalvular pressure, and compressive forces in the ways to new treatments. In this current opinion piece, we coaptation zone. This complex loading regime is cycli- examine the state of our knowledge about the (mal)adaptive cally repeated with every heartbeat for billions of times mechanisms (physiological and pathological growth and throughout our lifetime [1,2]. Ostensibly, these loading remodeling) of the atrioventricular heart valves. Furthermore, modes determine the valves’ microstructure and we review the evidence that suggests that valve maladaptation consequently their mechanical properties [3]. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -

Heart Valve Disease: Mitral and Tricuspid Valves
Heart Valve Disease: Mitral and Tricuspid Valves Heart anatomy The heart has two sides, separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen- rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves that regulate blood flow. The upper chambers are called the left and right atria, and the lower chambers are called the left and right ventricles. The mitral valve is located on the left side of the heart, between the left atrium and the left ventricle. This valve has two leaflets that allow blood to flow from the lungs to the heart. The tricuspid valve is located on the right side of the heart, between the right atrium and the right ventricle. This valve has three leaflets and its function is to Cardiac Surgery-MATRIx Program -1- prevent blood from leaking back into the right atrium. What is heart valve disease? In heart valve disease, one or more of the valves in your heart does not open or close properly. Heart valve problems may include: • Regurgitation (also called insufficiency)- In this condition, the valve leaflets don't close properly, causing blood to leak backward in your heart. • Stenosis- In valve stenosis, your valve leaflets become thick or stiff, and do not open wide enough. This reduces blood flow through the valve. Blausen.com staff-Own work, CC BY 3.0 Mitral valve disease The most common problems affecting the mitral valve are the inability for the valve to completely open (stenosis) or close (regurgitation). -
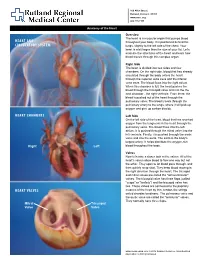
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Selecting Candidates for Transcatheter Mitral Valve Repair
• INNOVATIONS Key Points Selecting Candidates for • Mitral valve regurgitation, or leaky mitral valve, is a common valve disorder Transcatheter Mitral Valve Repair in which the leaflets of the mitral valve fail to seal effectively, resulting in Annapoorna S. Kini, MD Samin K. Sharma, MD some blood flowing back in the left atrium every time Mitral valve regurgitation is a common valve disorder The EVEREST (Endovascular Valve Edge-to- the left ventricle contracts. that causes blood to leak backward through the Edge Repair Study) II Trial was a randomized study This condition has been mitral valve and into the left atrium as the heart comparing the transcatheter approach using traditionally addressed muscle contracts. Mitral regurgitation can originate MitraClip®—a tiny cobalt chromium clip that sutures with open heart surgery. from degenerative or structural defects due to the anterior and posterior mitral valve leaflets—with aging, infection, or congenital anomalies. In contrast, surgery in patients with moderate to severe mitral • We use the latest imaging functional mitral regurgitation occurs when coronary regurgitation who are candidates for either procedure. techniques both to ensure artery disease or events such as a heart attack change that each patient is a good After five years, the study has demonstrated that the size and shape of the heart muscle, preventing candidate for the procedure, MitraClip was associated with a similar risk of death the mitral valve from opening and closing properly. In and to monitor their progress compared with mitral valve surgery after excluding people with moderate to severe mitral regurgitation, once the device is implanted. patients who required surgery within six months. -

Ventricular Anatomy for the Electrophysiologist (Part
Ventricular Anatomy for the REVIEW Electrophysiologist (Part II) SPECIAL 서울대학교 의과대학 병리학교실 서정욱 이화여자대학교 의학전문대학원 김문영 ABSTRACT The conduction fibers and Purkinje network of the ventricular myocardium have their peculiar location and immuno-histochemical characteristics. The bundle of His is located at the inferior border of the membranous septum, where the single trunk ramifies into the left and right bundle branches. The left bundle branches are clearly visible at the surface. The right bundles are hidden in the septal myocardium and it is not easy to recognize them. The cellular characters of the conduction bundles are modified myocardial cells with less cytoplasmic filaments. Myoglobin is expressed at the contractile part, whereas CD56 is expressed at the intercalated disc. A fine meshwork of synaptophysin positive processes is noted particularly at the nodal tissue. C-kit positive cells are scattered, but their role is not well understood. Purkinje cells are a peripheral continuation of bundles seen at the immediate subendocardium of the left ventricle. Key words: ■ conduction system ■ Purkinje network ■ pathology ■ arrhythmia ■ electrophysiology Introduction human heart. In this brief review, the histological characteristics of conduction cells, stained by The functional assessment of abnormal cardiac conventional and immuno-histochemical staining, are 3 rhythm and a targeted treatment based on demonstrated in the second part of the review. electrophysiologic studies are successful advances in cardiology.1 Morphological assessment or confirmation The characteristic location of the ventricular of hearts with such abnormalities is rare, not only due conduction system to the limited availability of human hearts but also inherent technological limitations of existing The atrioventricular node is situated in its technology.2 Classical morphological approaches and subendocardial location at the triangle of Koch. -

Transcatheter Aortic Valve Replacement
What is TAVR? Cardiac Catheterization: Important things to know that will help you get ready Transcatheter Aortic Valve Replacement (TAVR) is a procedure Your doctor will tell if you need to stop eating or drinking to fix the aortic valve without taking out the old valve. A TAVR before your procedure. Your doctor also will tell you if you does not need open heart surgery and the heart does not need must stop taking any medications before the procedure. to be stopped. Catheterization Lab In the Pre-Operative (Pre-Op) Room before your The surgeon puts a catheter (thin tube) into an artery in your Cardiac Catheterization upper leg or through a small cut in your chest. The catheter will • You will wear a hospital gown. We will ask you to take off all Transcatheter Aortic Valve carry a new valve to your heart. your clothing (even underwear), jewelry, dentures, glasses, Replacement (TAVR) hearing aids, etc. • An intravenous line (IV) may be put into a vein in your arm • We will prepare and clean the catheter site (where the catheter goes into your body). We will clean your skin with a special wash that kills germs. We may need to trim body hair. • We will ask you to empty your bladder (pee) before your procedure After Your Cardiac Catheterization • You may be on bed rest (lying flat) for 2 to 6 hours. To lower the risk of bleeding, we do not want you to bend your body at the catheter site (where the catheter went into your body) • Your nurse will often check your vital signs (blood pressure, heart rate, temperature) and catheter site • You must use a urinal or bed pan until you can safely stand and walk to the bathroom • While you are healing, do not do strenuous exercise (such as running or lifting weights).