Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WPW: WOLFF-PARKINSON-WHITE Syndrome
WPW: WOLFF-PARKINSON-WHITE Syndrome What is Wolff-Parkinson-White Syndrome? Wolff-Parkinson-White Syndrome, or WPW, is named for three physicians who described a syndrome in 1930 in young people with episodes of heart racing and an abnormal pattern on their electrocardiogram (ECG or EKG). Over the next few decades, it was discovered that this ECG pattern and the heart racing was due to an extra electrical pathway in the heart. Thus, WPW is a syndrome associated with an abnormal heart rhythm, or “arrhythmia”. Most people with WPW do not have any other problems with their heart. Normally, the electrical impulses in the heart originate in the atria or top chambers of the heart and spread across the atria. The electrical impulses are then conducted to the ventricles (the pumping/bottom chambers of the heart) through a group of specialized cells called the atrioventricular node or AV node. This is usually the only electrical pathway between the atria and ventricles. In WPW, there is an additional pathway made up of a few extra cells left over from when the heart formed. The conduction of electricity through the heart causes the contractions which are the “heartbeat”. What is WPW Syndrome as opposed to a WPW ECG? A person has WPW Syndrome if they experience symptoms from abnormal conduction through the heart by the WPW pathway. Most commonly, the symptom is heart racing, or “palpitations”. The particular type of arrhythmia in WPW is called “supraventricular tachycardia” or SVT. “Tachycardia” means fast heart rate; “supraventricular” means the arrhythmia requires the cells above the ventricles to be part of the abnormal circuit. -

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Blood Flow DHO8 7.8, Pg
Blood Flow DHO8 7.8, pg. 190 HS1/2017-2018 Circuits •Pulmonary circuit –The blood pathway between the right of the heart, to the lungs, and back to the left side of the heart. •Systemic circuit –The pathway between the left side of the heart, to the body, and back to the right side of the heart. The Pathway of Blood •Superior & Inferior Vena •Left Atrium Cava •Mitral Valve •Right Atrium •Left Ventricle •Tricuspid Valve •Aortic Semilunar Valve •Right Ventricle •Aorta •Pulmonary Semilunar -Arteries Valve -Arterioles •Pulmonary Artery -Capillaries •Lungs -Venules –Pulmonary Arterioles -Veins –Pulmonary Capillaries –Pulmonary Venules •Pulmonary Vein Blood Flow Through Heart Do You Know? • When blood leaves the left atrium, where does it go next? a) Aorta b) Left ventricle c) Right atrium d) Pulmonary artery And the answer is….A Do You Know? • After blood leaves the right atrium, what valve prevents the back flow? a) Pulmonary b) Mitral c) Tricuspid d) Aortic And the answer is…C Do You Know? • The right ventricle is the chamber of the heart that pumps blood for the pulmonary circulation. Based on this information, blood from the right ventricle is on its way to the _____. a) Liver b) Lungs c) Hands and feet And the answer is…B Do You Know? • Which of the following is correct order of blood flow for the right side of the heart? a) RA, Tricuspid valve, RV, PSLV, pulmonary artery b) RA, PSLV, RV, Tricuspid valve, pulmonary artery c) RA, Tricuspid valve, RV, pulmonary artery , PSLV And the answer is…A Do You Know? • Which of the following is correct order of blood flow for the left side of the heart? a) LA, Bicuspid valve, LV, ASLV, aorta b) LA, ASLV, LV, Bicuspid valve, aorta c) LA, Bicuspid valve, LV, ASLV, aorta And the answer is…C. -

Mitral Valve Prolapse
MITRAL VALVE PROLAPSE WHAT IS MITRAL VALVE PROLAPSE? The mitral valve is a heart valve with two tissue flaps, called leaflets, that open and close. It is located between the left upper chamber (atrium) and left lower chamber (ventricle) of the heart. Mitral valve prolapse occurs when the mitral valve bulges into the left atrium when the heart contracts (squeezes). This may keep the leaflets from closing properly. The result is a backward flow of blood into the left atrium. This is referred to as leaking or regurgitation. Most of the time, however, mitral valve prolapse causes no symptoms and no problems. WHAT ARE THE SYMPTOMS? Most people with mitral valve prolapse have no symptoms. However, some have brief periods of rapid heartbeat or skipped beats. Some people have sharp chest pains lasting seconds or minutes. Some people have shortness of breath when climbing stairs or with heavy exertion. You may notice symptoms more when you are physically active HOW IS IT DIAGNOSED? Often mitral valve prolapse is discovered during a physical examination, when the doctor listens to your heart with a stethoscope. The valve leaflets create a “click” sound that the doctor may hear. If the valve leaks, the doctor may also hear a murmur. The doctor may ask you to stand, sit, lie down, or squat during your exam, so he or she can better hear the heart sounds. An echocardiogram passes sound waves through the heart to create an image. It is the best test to diagnose mitral valve prolapse. The images show the prolapse, any thickening of the valve leaflets, and any leakage of blood through the prolapsed valve. -
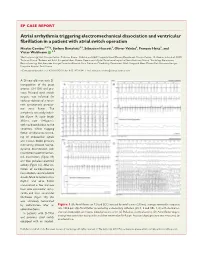
Atrial Arrhythmia Triggering Electromechanical Dissociation And
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Cardiac Arrest and Ventricular Fibrillation * a Method of Treatment by Electrical Shock
Thorax: first published as 10.1136/thx.7.3.205 on 1 September 1952. Downloaded from Thorax (1952), 7, 205. CARDIAC ARREST AND VENTRICULAR FIBRILLATION * A METHOD OF TREATMENT BY ELECTRICAL SHOCK BY I. K. R. MCMILLANt F. B. COCKETT, AND P. STYLES Froml the Departnment of Cardiology, tile Professorial Surgical Unit, and tile Departalzent of Phlysical Medicine, St. Thomlas's Hospital, London (RECEIVED FOR PUBLICATION APRIL 30, 1952) Cardiac arrest during operation is a subject of VENTRICULAR FIBRILLATION importance to all surgeons. During intra-thoracic The treatment of ventricular fibrillation bv operations cardiac arrest and ventricular fibrilla- electrical shock therapy is not new and was first tion can be differentiated by direct observation of tried experimentally in 1899 (Prevost and Battelli). the heart. In recent years this method has been extensively Ventricular fibrillation is an incoordinated type investigated in the United States and France of contraction which produces no useful beats. (Kouwenhoven and Kay, 1951; Johnson and The typical rippling movements of the ventricular Kirby, 1951 ; Santy and Marion, 1950). muscle are unmistakable when seen or felt with The treatment of ventricular fibrillation by the hand directly on the heart. The treatment of zlectrical shock gives encouraging results in the copyright. a heart that has stopped is cardiac massage and experimental animal, and may be a useful adjunct adequate oxygenation. The treatment of ventri- in the operating theatre during cardiac and general cular fibrillation which we recommend is, first, surgery. Under these conditions shock therapy massage to maintain the oxygen supply to the can be rapidly applied. Provided that adequate heart through the coronary arteries, followed oxygenation is maintained and direct cardiac mas- http://thorax.bmj.com/ rapidly by electrical shocking to restore normal sage promptly initiated and maintained, shock rhythm therapy can be applied without undue haste. -

Mitral Valve Prolapse Page 1 of 3
Mitral valve prolapse Page 1 of 3 Mitral valve prolapse Definition Mitral valve prolapse is a heart problem in which the valve that separates the upper and lower chambers of the left side of the heart does not close properly. Alternative Names Barlow syndrome; Floppy mitral valve; Myxomatous mitral valve; Billowing mitral valve; Systolic click-murmur syndrome; Prolapsing mitral leaflet syndrome Causes The mitral valve helps blood on the left side of the heart flow in one direction. It closes to keep blood from moving backwards when the heart beats (contracts). Mitral valve prolapse is the term used when the valve does not close properly. It can be caused by many different things. In most cases, it is harmless and patients usually do not know they have the problem. As much as 10% of the population has some minor, insignificant form of mitral valve prolapse, but it does not generally affect their lifestyle. In a small number of cases, the prolapse can cause blood to leak backwards. This is called mitral regurgitation. Mitral valves that are structurally abnormal can raise the risk for bacterial infection. Some forms of mitral valve prolapse seem to be passed down through families (inherited). Mitral valve prolapse has been associated with Graves disease . Mitral valve prolapse often affects thin women who may have minor chest wall deformities, scoliosis , or other disorders. Mitral valve prolapse is associated with some connective tissue disorders, especially Marfan syndrome . Other conditions include: • Ehlers-Danlos syndrome • Osteogenesis imperfecta • Polycystic kidney disease http://eclinicalworks.adam.com/content.aspx?ref=applications.adam.com&url=eclinicalwo .. -

Basic Arrhythmia Review Guide-Advanced
BASIC ARRHYTHMIA REVIEW GUIDE ADVANCED The following study guide provides a review of information covered in the basic arrhythmia competency. Preparation with this guide will help to achieve success on the exam. Sample questions and websites are provided at the end of this guide. DESCRIPTION OF THE HEART The adult heart is a muscular organ weighing less than a pound and about the size of a clenched fist. It lies between the right and Left left lung in an area called the mediastinal cavity behind the sternum of the breast bone. Approximately two-thirds of the heart Atrium lies to the left of the sternum and one-third to the right of the sternum. Right HEART MUSCLES Atrium The heart is composed of three layers each with its own special function. The outermost layer is called the pericardium, essentially a sac around the heart. The middle and thickest layer of the heart is called the Left myocardium. This layer contains all the atrial and ventricular Ventricle muscle fibers needed for contraction as well as the blood supply Right and electrical conduction system. Ventricle The innermost layer of the heart is the endocardium and is composed of endothelium and connective tissue. Any disruption or injury to this endothelium can lead to infection, which in turn can cause valve damage, sepsis, or death. CHAMBERS A normal human heart contains four separate chambers: right atrium, left atrium, right ventricle, and left ventricle. The right and left sides of the heart are divided by a septum. The right atrium (RA) receives oxygen-poor (venous) blood from the body’s organs via the superior and inferior vena cava (SVC and IVC). -

The Refractory VF Arrest Patient: a Review of the Current Treatment
1/24/2018 The Refractory VF Arrest Patient: A Review of the Current Treatment Options Marc Conterato, MD, FACEP Office of the Medical Director North Memorial Health Ambulance Service DISCLOSURE STATEMENT • Board Member, MN Resuscitation Consortium - Images of any commercial devices or medications are for illustration purposes only. The inclusion of such images in this presentation does not imply endorsement of any specific device or company. 2 Objectives • Delineate Refractory Ventricular Fibrillation (RVF) • Recurrent VF versus Refractory VF • What is “Electrical Storm” • Current Pre-hospital treatments • Additional hospital treatments • Dual/Double Sequential Defibrillation • ECMO/ECLS-A New Hope? 3 1 1/24/2018 A Standard Scenario • 55 YOF collapses at stop light, and her car rolls into the car in front of her. Airbags do not deploy, and bystanders find her slumped over the steering wheel and pulseless. • First responder/bystanders start CPR, and deliver three AED shocks prior to EMS arrival. • On EMS arrival, a fourth shock is delivered, an IO and alternative airway placed. Automated CPR started. • Epinephrine 2 mg (total) and Amiodarone 300 mg given, and the patient remains in VF. • Patient downtime is now ~25 minutes, and repeat evaluation reveals persistent VF. 4 What are your options • Continue CPR for a total of 30 minutes with recurrent defibrillations, additional epinephrine, bicarbonate and Amiodarone? • “Load and Go” to the local hospital with CPR enroute and continuing the resuscitation? • “Load and Go” to the local CCL (Cardiac Cath Lab) hospital with CPR enroute and continuing the resuscitation, with possible PCI with ongoing CPR? • Call HEMS unit for transfer to CCL hospital, but can they continue effective CPR in the helicopter? • “Load and Go” to a ECMO/ECLS center with CPR enroute and continuing the resuscitation, on the basis they can accept the patient and continue resuscitation? 5 Defining the problem: What is recurrent versus refractory VF (RVF)? • Recurrent VF is a rhythm that terminates with cardioversion, but then recurs rapidly. -

Tachycardia (Fast Heart Rate)
Tachycardia (fast heart rate) Working together to improve the diagnosis, treatment and quality of life for all those aff ected by arrhythmias www.heartrhythmalliance.org Registered Charity No. 1107496 Glossary Atrium Top chambers of the heart that receive Contents blood from the body and from the lungs. The right atrium is where the heart’s natural pacemaker (sino The normal electrical atrial node) can be found system of the heart Arrhythmia An abnormal heart rhythm What are arrhythmias? Bradycardia A slow heart rate, normally less than 60 beats per minute How do I know what arrhythmia I have? Cardiac Arrest the abrupt loss of heart function, breathing and consciousness Types of arrhythmia Cardioversion a procedure used to return an abnormal What treatments are heartbeat to a normal rhythm available to me? Defi brillation a treatment for life-threatening cardiac arrhythmias. A defibrillator delivers a dose of electric current to the heart Important information This booklet is intended for use by people who wish to understand more about Tachycardia. The information within this booklet comes from research and previous patients’ experiences. The booklet off ers an explanation of Tachycardia and how it is treated. This booklet should be used in addition to the information given to you by doctors, nurses and physiologists. If you have any questions about any of the information given in this booklet, please ask your nurse, doctor or cardiac physiologist. 2 Heart attack A medical emergency in which the blood supply to the heart is blocked, causing serious damage or even death of heart muscle Tachycardia Fast heart rate, more than 100 beats per minute Ventricles The two lower chambers of the heart. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells.