Anatomy of the True Interatrial Septum for Transseptal Access to the Left Atrium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Abnormally Enlarged Singular Thebesian Vein in Right Atrium
Open Access Case Report DOI: 10.7759/cureus.16300 Abnormally Enlarged Singular Thebesian Vein in Right Atrium Dilip Kumar 1 , Amit Malviya 2 , Bishwajeet Saikia 3 , Bhupen Barman 4 , Anunay Gupta 5 1. Cardiology, Medica Institute of Cardiac Sciences, Kolkata, IND 2. Cardiology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 3. Anatomy, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 4. Internal Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 5. Cardiology, Vardhman Mahavir Medical College (VMMC) and Safdarjung Hospital, New Delhi, IND Corresponding author: Amit Malviya, [email protected] Abstract Thebesian veins in the heart are subendocardial venoluminal channels and are usually less than 0.5 mm in diameter. The system of TV either opens a venous (venoluminal) or an arterial (arterioluminal) channel directly into the lumen of the cardiac chambers or via some intervening spaces (venosinusoidal/ arteriosinusoidal) termed as sinusoids. Enlarged thebesian veins are reported in patients with congenital heart disease and usually, multiple veins are enlarged. Very few reports of such abnormal enlargement are there in the absence of congenital heart disease, but in all such cases, they are multiple and in association with coronary artery microfistule. We report a very rare case of a singular thebesian vein in the right atrium, which was abnormally enlarged. It is important to recognize because it can be confused with other cardiac structures like coronary sinus during diagnostic or therapeutic catheterization and can lead to cardiac injury and complications if it is attempted to cannulate it or pass the guidewires. -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -
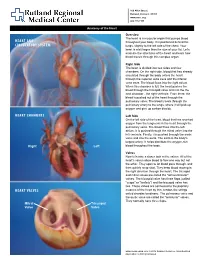
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities. -

The American Society of Echocardiography
1 THE AMERICAN SOCIETY OF ECHOCARDIOGRAPHY RECOMMENDATIONS FOR CARDIAC CHAMBER QUANTIFICATION IN ADULTS: A QUICK REFERENCE GUIDE FROM THE ASE WORKFLOW AND LAB MANAGEMENT TASK FORCE Accurate and reproducible assessment of cardiac chamber size and function is essential for clinical care. A standardized methodology creates a common approach to the assessment of cardiac structure and function both within and between echocardiography labs. This facilitates better communication and improves the ability to compare results between studies as well as differentiate normal from abnormal findings in an individual patient. This document summarizes key points from the 2015 ASE Chamber Quantification Guideline and is meant to serve as quick reference for sonographers and interpreting physicians. It is designed to provide guidance on chamber quantification for adult patients; a separate ASE Guidelines document that details recommended quantification methods in the pediatric age group has also been published and should be used for patients <18 years of age (3). (1) For details of the methodology and the rationale for current recommendations, the interested reader is referred to the complete Guideline statement. Figures and tables are reproduced from ASE Guidelines. (1,2) Table of Contents: 1. Left Ventricle (LV) Size and Function p. 2 a. LV Size p. 2 i. Linear Measurements p. 2 ii. Volume Measurements p. 2 iii. LV Mass Calculations p. 3 b. Left Ventricular Function Assessment p. 4 i. Global Systolic Function Parameters p. 4 ii. Regional Function p. 5 2. Right Ventricle (RV) Size and Function p. 6 a. RV Size p. 6 b. RV Function p. 8 3. Atria p. -

The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S
The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S. Balto, Steven A. Howard, BMEn., Paul A. Iaizzo, Ph.D.2 Departments of Biomedical Engineering1 and Surgery2 University of Minnesota, Minneapolis, MN 55455 Introduction Apparatus Conclusion Approximately 1 in 4 humans on this earth is born with a congenital heart •The apparatus designed for this study was great for initial testing of the defect that involves the failure of the complete formation of the fossa ovalis tissue properties of the fossa ovalis of large mammalian hearts, either of membrane between the left and right atria1. This defect, better known as a PFO research animals or of human donor hearts. However, physiological or Patent Fossa Ovalis is an opening in the fossa ovalis membrane that results properties weren’t really observed in the testing and so that is a starting from the failure of the fetal structures (septum primum and septum secundum) point for advancing the quality of this research. The ability for the system to from closing together at the moment of birth2. The opening can be classified in have interchangeable depression, puncture, and dilation devices adds to the a variety of ways, but the most common are “shunts” which cause blood to flexibility of the current setup. The visualization of the inside of the heart flow from one atria to the other. A shunt that results in the blood flowing from adds a new dimension to this research as well since it is able to visualize all the left to right atria will cause the blood pressure to increase in the right side cardiac structures under ~10 mmHg of pressure. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition
biomolecules Article Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition 1, 2, 1 1 Luca Sgarra y, Alessandro Santo Bortone y, Maria Assunta Potenza , Carmela Nacci , Maria Antonietta De Salvia 1, Tommaso Acquaviva 2, Emanuela De Cillis 2, Marco Matteo Ciccone 2, Massimo Grimaldi 3 and Monica Montagnani 1,* 1 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (L.S.); [email protected] (M.A.P.); [email protected] (C.N.); [email protected] (M.A.D.S.) 2 Department of Emergency and Organ Transplantation—Section of Cardiovascular Diseases, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (A.S.B.); [email protected] (T.A.); [email protected] (E.D.C.); [email protected] (M.M.C.) 3 General Hospital “F. Miulli” Acquaviva delle Fonti, 70021 Bari, Italy; fi[email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 28 March 2020; Accepted: 2 June 2020; Published: 4 June 2020 Abstract: We explored the significance of the L-Arginine/asymmetric dimethylarginine (L-Arg/ADMA) ratio as a biomarker of endothelial dysfunction in stroke patients. To this aim, we evaluated the correlation, in terms of severity, between the degree of endothelial dysfunction (by L-Arg/ADMA ratio), the methylene tetrahydrofolate reductase (MTHFR) genotype, and the interatrial septum (IAS) phenotype in subject with a history of stroke. Methods and Results: L-Arg, ADMA, and MTHFR genotypes were evaluated; the IAS phenotype was assessed by transesophageal echocardiography.