Premature Obliteration of the Foramen Ovale by G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter Xi the Circulatory System and Blood
CHAPTER XI THE CIRCULATORY SYSTEM AND BLOOD Page General characterlstlcs______ __ __ _ __ __ __ __ __ __ _ 239 of these organs are independent of the beating of The pericardium ___ __ __ __ 239 the principal heart, and their primary function is The heart. _____ __ __ 240 Physiology of the heart.______________________________________________ 242 to oscillate the blood within the pallial sinuses. Automatism of heart beat. _ 242 The pacemaker system_ 245 THE PERICARDIUM Methods of study of heart beat_____________________________________ 247 Frequency of beat___ __ __ _ 248 Extracardlac regulatlon____ __ __ _ 250 The heart is located in the pericardium, a thin Effects of mineral salts and drugs___________________________________ 251 Blood vessels_ __ ___ _ 253 walled chamber between the visceral mass and the The arterial system______ __ __ ___ __ __ __ __ __ 253 adductor muscle (fig. 71). In a live oyster the The venous system_________________________________________________ 254 location of the heart is indicated by the throbbing The accessory heart._____________ 258 The blood______ __ __ __ __ __ __ __ 259 of the wall of the pericardium on the left side. Color of blood_ __ __ 261 Here the pericardium wall lies directly under the The hyaline cells___________________________________________________ 261 The granular cells .______________________________________ 262 shell. On the right side the promyal chamber Specific gravity of blood____________________________________________ 265 extends down over the heart region and the mantle Serology ___ __________ __________________ ____ __ ______________________ 265 Bibliography __ __ __ __ __ __ __ 266 separates the pericardium wall from the shell. The cavity in which the heart is lodged is slightly A heart, arteries, veins, and open sinuses form asymmetrical; on the right side it extends farther the circulatory system of oysters and other bi along the anterior part of the adductor muscle valves. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -
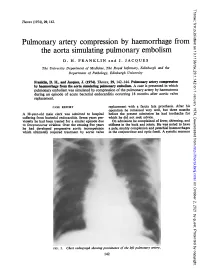
The Aorta Simulating Pulmonary Embolism D
Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. Downloaded from Thorax (1974), 29, 142. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism D. H. FRANKLIN and J. JACQUES The University Department of Medicine, The Royal Infirmary, Edinburgh and the Department of Pathology, Edinburgh University Franklin, D. H., and Jacques, J. (1974). Thorax, 29, 142-144. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism. A case is presented in which pulmonary embolism was simulated by compression of the pulmonary artery by haematoma during an episode of acute bacterial endocarditis occurring 18 months after aortic valve replacement. CASE REPORT replacement with a fascia lata prosthesis. After his operation he remained very well, but three months A 38-year-old male clerk was admitted to hospital before the present admission he had toothache for suffering from bacterial efndocarditis. Seven years pre- which he did not seek advice. viomly he had been treated for a similar episode due On admission he complained of fever, shivering, and to Streptococcus viridans. Over the ensuing five years stiffness in the back and joints. He was noted to have he had developed progressive aortic incompetence a pale, muddy complexion and petechial haemorrhages which ultimately required treatment by aortic valve in the conjunctivae and optic fundi. A systolic murmur http://thorax.bmj.com/ on October 2, 2021 by guest. Protected copyright. FIG. 1. Chesvt radiograph showing prominence of the left pulmonary artery. 142 Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -

The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S
The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S. Balto, Steven A. Howard, BMEn., Paul A. Iaizzo, Ph.D.2 Departments of Biomedical Engineering1 and Surgery2 University of Minnesota, Minneapolis, MN 55455 Introduction Apparatus Conclusion Approximately 1 in 4 humans on this earth is born with a congenital heart •The apparatus designed for this study was great for initial testing of the defect that involves the failure of the complete formation of the fossa ovalis tissue properties of the fossa ovalis of large mammalian hearts, either of membrane between the left and right atria1. This defect, better known as a PFO research animals or of human donor hearts. However, physiological or Patent Fossa Ovalis is an opening in the fossa ovalis membrane that results properties weren’t really observed in the testing and so that is a starting from the failure of the fetal structures (septum primum and septum secundum) point for advancing the quality of this research. The ability for the system to from closing together at the moment of birth2. The opening can be classified in have interchangeable depression, puncture, and dilation devices adds to the a variety of ways, but the most common are “shunts” which cause blood to flexibility of the current setup. The visualization of the inside of the heart flow from one atria to the other. A shunt that results in the blood flowing from adds a new dimension to this research as well since it is able to visualize all the left to right atria will cause the blood pressure to increase in the right side cardiac structures under ~10 mmHg of pressure. -
Cardiovascular System: Early Development
CARDIOVASCULAR SYSTEM: EARLY DEVELOPMENT - I CARDIOVASCULAR SYSTEM: EARLY DEVELOPMENT - II HEART AND ITS NEIGHBORHOOD- I HEART AND ITS NEIGHBORHOOD- II BLOOD VESSELS OF THE EMBRYO (at 26 days) FROM TUBE TO FOUR CHAMBERS EXTERNAL VIEW NORMAL : Loop to the RIGHT: Levocardia! ABNORMAL: Loop to the LEFT: Dextrocardia! FROM TUBE TO FOUR CHAMBERS INTERNAL VIEW FOUR CHAMBERS- ULTRASOUND VIEW @ 20 wks ATRIAL SEPTUM FORMATION- I ATRIAL SEPTUM FORMATION- II ATRIAL SEPTUM FORMATION- III ATRIAL SEPTUM FORMATION- IV ATRIAL SEPTUM FORMATION- V THE DEFINITIVE RIGHT ATRIUM **NOTE: In 25% of normal population, the foramen ovale remains ‘probe patent’. THE DEFINITIVE LEFT ATRIUM ATRIAL SEPTAL DEFECTS- “Fossa Ovalis” type ATRIAL SEPTAL DEFECTS- Unrelated to Foramen Ovale AORTIC ARCHES AND DERIVATIVES - I AORTIC ARCHES AND DERIVATIVES - II RECURRENT LARYNGEAL NERVES Right vs Left PATENT DUCTUS ARTERIOSUS Prostaglandin: Keeps the duct Patent Indomethacin: Closes the duct. RIGHT AORTIC ARCH: Mirror image branching ABERRANT RIGHT SUBCLAVIAN ARTERY: Occurs in 0.5% of people. Usually asymptomatic. DOUBLE AORTIC ARCH: “Vascular ring” Causes airway obstruction, stridor in infancy. COARCTATION OF THE AORTA THE CARDINAL VEINS AND THE VENAE CAVAE THE CARDINAL VEINS AND THE VENAE CAVAE SINUS VENOSUS AND THE CORONARY SINUS PERSISTENT LEFT SVC 0.3% of general population. 4 % of patients with Cong. Ht Dis. Usually drains to Coronary sinus. Usually asymptomatic. Enlarged coronary sinus is a clue. Left SVC to coronary sinus UMBILICAL AND VITELLINE VEINS- I: Liver, portal vein and ductus venosus. © 2005 Elsevier UMBILICAL AND VITELLINE VEINS- II: Liver, portal vein and ductus venosus. LYMPHATIC SYSTEM- I LYMPHATIC SYSTEM- II FETAL CIRCULATION POSTNATAL CIRCULATION. -

Unit 1 Lecture 2
Unit 1 Lecture 2 Unit 1 Lecture 2 THE HEART Anatomy of the Heart The heart rests on the diaphragm in a space called the mediastinum. It weighs @ 300 grams and is about as big as a clenched fist. The pointed end is called the apex and the opposite end is called the base but is really the top of the heart. The bulk of the heart tissue is made up of the left ventricle. A pericardium (a 3-layered bag) surrounds the heart and composed of fibrous pericardium (that provides protection to the heart, prevents overstretching, and anchors the heart in the mediastinum), a serous pericardium which is composed of two layers (the parietal layer or outer layer that is fused to the fibrous pericardium and the visceral layer or the inner layer that adheres to heart muscle). The visceral pericardium is also called the epicardium. Pericardial fluid is found in the pericardial cavity, which is located between the two layers, helps lubricate and reduces friction between membranes as the heart moves. The heart wall is composed of three layers: the epicardium (see visceral pericardium above), the myocardium which is cardiac muscle tissue and the bulk of mass in the heart, and endocardium or the innermost layer of the heart. This lining is continuous through all of the blood vessels except for the capillaries. The Heart Chambers and Valves Internally the heart is divided into four chambers. The two upper chambers are the right and left atria. Each has an appendage (auricle) whose function is to increase the volume of the atria. -

The Cardiovascular System
11 The Cardiovascular System WHAT The cardiovascular system delivers oxygen and HOW nutrients to the body tissues The heart pumps and carries away wastes blood throughout the body such as carbon dioxide in blood vessels. Blood flow via blood. requires both the pumping action of the heart and changes in blood pressure. WHY If the cardiovascular system cannot perform its functions, wastes build up in tissues. INSTRUCTORS Body organs fail to function properly, New Building Vocabulary and then, once oxygen becomes Coaching Activities for this depleted, they will die. chapter are assignable in hen most people hear the term cardio- only with the interstitial fluid in their immediate Wvascular system, they immediately think vicinity. Thus, some means of changing and of the heart. We have all felt our own “refreshing” these fluids is necessary to renew the heart “pound” from time to time when we are ner- nutrients and prevent pollution caused by vous. The crucial importance of the heart has been the buildup of wastes. Like a bustling factory, the recognized for ages. However, the cardiovascular body must have a transportation system to carry system is much more than just the heart, and its various “cargoes” back and forth. Instead of from a scientific and medical standpoint, it is roads, railway tracks, and subways, the body’s important to understand why this system is so vital delivery routes are its hollow blood vessels. to life. Most simply stated, the major function of the Night and day, minute after minute, our tril- cardiovascular system is transportation. Using lions of cells take up nutrients and excrete wastes. -

The Surgical Treatment of Constrictive Fibrous Endocarditis
The Surgical Treatment of Constrictive Fibrous Endocarditis CH. DUBOST, P. MAURICE, A. GERBAUX, E. BERTRAND, R. RULLIERE, F. VIAL, A. BARRILLON, C. PRIGENT, A. CARPENTIER, R. SOYER Constrictive fibrous endocarditis is a pathological entity described From the Department of Cardiovascular Surgery, by Lo6ffler in 1936. Its etiology is unknown. The clinical course Broussais Hospital, Paris, France is characterized by an evolution towards cardiac insufficiency leading rapidly to a fatal outcome. Modern paraclinical investi- gations are necessary to assess the diagnostic. Cardiac catheteriza- tion brings the proof of adiastole and angiocardiography re- Endocardiectomy was first performed by us in 19715-7 veals the shape of amputation of the ventricle with auriculo- and the patient was improved. Since then, four more cases ventricular regurgitation. The operative procedure consists of have been done, either right or left sided or combined resection of the ventricular fibrosis including the valves and endocardiectomy and there is no doubt that a better auriculo-ventricular valve replacement 'by ;a prosthetic valve. The disease affects both Caucasians and Negros. Our experience knowledge of the clinical aspects of the disease will includes 5 cases. The indications for operation and their results increase the number of cases referred for treatment. are discussed. Pathology C ONSTRICTIVE FIBROUS ENDOCARDITIS, first described The development of fibrous endocarditis is similar in by Loeffler6 in 1936, consists of endocardial fibrosis all cases: it involves the filling chambers of one or several millimeters in thickness which is potentially both ventricles including the papillary muscles and constrictive and limited to the ventricular cavities. chordae tendinae of the mitral and tricuspid valves The clinical course is characterized by an evolution which themselves are included in the process in the towards cardiac insufficiency which may be predomi- majority of cases.8 nantly right or left sided leading rapidly to a fatal outcome. -

THE HEART Study Objectives
THE HEART Study Objectives: You are responsible to understand the basic structure of the four chambered heart, the position and structure of the valves, and the path of blood flow through the heart. Structure--The Outer Layer pericardium: a specialized name for the celomic sac that surrounds the heart. fibrous pericardium: the most outer, fibrous layer of the pericardium. serous pericardium: there are two surfaces (layers) to this tissue a) parietal layer: the outer layer of the serosa surrounding the heart. Lines the fibrous pericardium, and the combination of the two layers is also called the parietal pericardium. b) visceral layer: the inner layer of the serosa surrounding and adhering to the heart. Also called the visceral pericardium or epicardium. pericardial cavity: the potential space between the parietal and visceral layers of the serous pericardium. The cavity is filled with serous fluid Structure--The Heart: Blood is delivered to the right atrium from the coronary sinus, inferior vena cava and superior vena cava Blood travels to the right ventricle from the right atrium via the right atrioventricular valve Blood travels from the left atrium to the left ventricle via the left atrioventricular valve Blood leaves the heart via the pulmonary valve to the pulmonary trunk, is delivered to the lungs for oxygenation and returns via the pulmonary veins to the left atrium a) crista terminalis: separates the anterior rough-walled and posterior smooth-walled portions of the right atrium. b) right auricle: small, conical, muscular, pouch-like appendage. c) musculi pectinate (pectinate muscles): internal muscular ridges that fan out anteriorly from the crista terminalis. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5]. -

Download PDF File
Folia Morphol. Vol. 79, No. 4, pp. 736–741 DOI: 10.5603/FM.a2020.0009 O R I G I N A L A R T I C L E Copyright © 2020 Via Medica ISSN 0015–5659 journals.viamedica.pl Development of the interatrial wall during the ontogenesis of foetuses and children up to one year of age R. Kamiński1, A. Kosiński1, A. Kaczyńska1, M. Zajączkowski1, G. Piwko1, M. Gleinert-Rożek1, T. Gos2, K. Karnecki2 1Department of Clinical Anatomy, Medical University of Gdansk, Poland 2Department of Forensic Medicine, Medical University of Gdansk, Poland [Received: 29 October 2019; Accepted: 30 December 2019] Background: The foramen ovale, present in foetal interatrial septum, plays an important role during foetal life. During delivery, foramen ovale closes and be- comes fossa ovalis, starting the pulmonary circulation. The aim of our study was to describe the growth of the interatrial wall and changes in location of the foramen ovale, and fossa ovalis during the ontogenesis in the human hearts. Materials and methods: The study was performed on post-mortem material obtained from 92 human hearts from 22nd week of foetal life up to 1 year of age, fixed in a 4% formalin solution. Results: The interatrial wall size in the studied development period was greater in the horizontal than in the vertical dimension. During ontogenesis up to 1 year old, the anterior and inferior parts of the interatrial wall increased their shares considerably by 8% and 6%, respectively. The percentage participation of foramen ovale in the interatrial wall construction in the foetal period formed more than 50% of its size and fairly decreased reaching in infants about 39%.