Chapter Xi the Circulatory System and Blood
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Aorta Simulating Pulmonary Embolism D
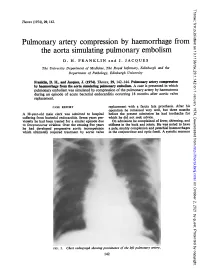
Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. Downloaded from Thorax (1974), 29, 142. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism D. H. FRANKLIN and J. JACQUES The University Department of Medicine, The Royal Infirmary, Edinburgh and the Department of Pathology, Edinburgh University Franklin, D. H., and Jacques, J. (1974). Thorax, 29, 142-144. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism. A case is presented in which pulmonary embolism was simulated by compression of the pulmonary artery by haematoma during an episode of acute bacterial endocarditis occurring 18 months after aortic valve replacement. CASE REPORT replacement with a fascia lata prosthesis. After his operation he remained very well, but three months A 38-year-old male clerk was admitted to hospital before the present admission he had toothache for suffering from bacterial efndocarditis. Seven years pre- which he did not seek advice. viomly he had been treated for a similar episode due On admission he complained of fever, shivering, and to Streptococcus viridans. Over the ensuing five years stiffness in the back and joints. He was noted to have he had developed progressive aortic incompetence a pale, muddy complexion and petechial haemorrhages which ultimately required treatment by aortic valve in the conjunctivae and optic fundi. A systolic murmur http://thorax.bmj.com/ on October 2, 2021 by guest. Protected copyright. FIG. 1. Chesvt radiograph showing prominence of the left pulmonary artery. 142 Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Structures of the Heart
Anatomy Tip Structures of the Heart In an effort to aid Health Information Management Coding Professionals for ICD-10, the following anatomy tip is provided with an educational intent. TIP: The Heart is made up of three main structures: 1. The Pericardium 2. The Heart Wall 3. The Chambers of the Heart The pericardium surrounds the heart. The outer layer, called the fibrous pericardium, secures the heart to surrounding structures like the blood vessels and the diaphragm. The inner layer, called the serous pericardium, is a double-layered section of the heart. Inside the two layers, serous fluid, known as pericardial fluid, lubricates and helps the heart move fluidly when beating. The heart wall is made up of three tissue layers: the epicardium, the myocardium, and the endocardium. The epicardium, which is the outermost layer, is also known as the visceral pericardium because it is also the inner wall of the pericardium. The middle layer, known as the myocardium, is formed out of contracting muscle. The endocardium, the innermost layer, covers heart valves and acts as a lining of the heart chambers. It is also in contact with the blood that is pumped through the heart, in order to push blood into the lungs and throughout the rest of the body. The four heart chambers are crucial to the heart’s function, composed of the atria, the right atrium and left atrium, and ventricles, the right ventricle and left ventricle. The right and left atria are thin-walled chambers responsible for receiving blood from veins, while the left and right ventricle are thick-walled chambers responsible for pumping blood out of the heart. -

Premature Obliteration of the Foramen Ovale by G
PREMATURE OBLITERATION OF THE FORAMEN OVALE BY G. AUSTIN GRESHAM From the Department of Pathology, University of Cambridge Received August 16, 1955 Obliteration of the foramen ovale occurring during intrauterine life is a rare condition. It throws some light on the mechanism of development of endocardial fibroelastosis and also upon the factors concerned in determining the size of the aorta and of the cardiac chambers. CASE HISTORY The patient was the second child of a mother (aet. 21) whose previous obstetric history was normal. Apart from two periods of rather rapid gain of weight for which no cause could be found, the pregnancy was uneventful. The child was eleven days postmature; labour was induced with an enema and lasted forty-five minutes. The infant cried lustily but was cyanosed: the heart was clinically normal. Abnormalities were present in all four limbs. The left radius and ulna were absent and rudimentary digits were present on the skin over the distal end of the limb. Terminal phalanges were absent in the fingers of the right hand, and four toes were present on each foot with a rudimentary fifth digit on the left foot. Cyanosis and dyspniea became more intense and the child died three hours after birth despite the use of oxygen. NECROPSY The body was that of a full-term male infant (weight 3430 g.). The limbs were abnormal as previously described. The lips were blue-black in colour. On the septal wall of the right atrium a hemispherical grey-white area (10x 12 x 4 mm. deep) with a central dimple filled in the usual site of the fossa ovalis (Fig. -

Cardiology Self Learning Package
Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Module 1: Anatomy and Physiology of the Heart Heart. Page 1 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) CONTENT Introduction…………………………………………………………………………………Page 3 How to use the ECG Self Learning package………………………………………….Page 4 Overview of the Heart…………………………………………………...…………..…….Page 5 Location, Size and Shape of the Heart…………………………………………………Page 5 The Chambers of the Heart…………….………………………………………..……….Page 7 The Circulation System……………………………………….………………..…………Page 8 The Heart Valve Anatomy………………………….…………………………..…………Page 9 Coronary Arteries…………………………………………….……………………..……Page 10 Coronary Veins…………………………………………………………………..……….Page 11 Cardiac Muscle Tissue……………………………………………………………..……Page 12 The Conduction System………………………………………………………………...Page 13 Cardiac Cycle……………………………………………………………………………..Page 15 References…………………………………………………………………………………Page 18 Module Questions………………………………………………………………………..Page 19 Module Evaluation Form………………………………………………………………..Page 22 [Module 1: Anatomy and Physiology of the Heart Page 2 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) INTRODUCTION Welcome to Module 1: Anatomy and Physiology of the Heart. This self leaning package is designed to as tool to assist nurse in understanding the hearts structure and how the heart works. The goal of this module is to review: Location , size and shape of the heart The chambers of the heart The circulation system of the heart The heart’s valve anatomy Coronary arteries and veins Cardiac muscle tissue The conduction system The cardiac cycle This module will form the foundation of your cardiac knowledge and enable you to understand workings of the heart that will assist you in completing other modules. Learning outcomes form this module are: To state the position of the heart, the size and shape. -

Unit 1 Lecture 2
Unit 1 Lecture 2 Unit 1 Lecture 2 THE HEART Anatomy of the Heart The heart rests on the diaphragm in a space called the mediastinum. It weighs @ 300 grams and is about as big as a clenched fist. The pointed end is called the apex and the opposite end is called the base but is really the top of the heart. The bulk of the heart tissue is made up of the left ventricle. A pericardium (a 3-layered bag) surrounds the heart and composed of fibrous pericardium (that provides protection to the heart, prevents overstretching, and anchors the heart in the mediastinum), a serous pericardium which is composed of two layers (the parietal layer or outer layer that is fused to the fibrous pericardium and the visceral layer or the inner layer that adheres to heart muscle). The visceral pericardium is also called the epicardium. Pericardial fluid is found in the pericardial cavity, which is located between the two layers, helps lubricate and reduces friction between membranes as the heart moves. The heart wall is composed of three layers: the epicardium (see visceral pericardium above), the myocardium which is cardiac muscle tissue and the bulk of mass in the heart, and endocardium or the innermost layer of the heart. This lining is continuous through all of the blood vessels except for the capillaries. The Heart Chambers and Valves Internally the heart is divided into four chambers. The two upper chambers are the right and left atria. Each has an appendage (auricle) whose function is to increase the volume of the atria. -

The Cardiovascular System
11 The Cardiovascular System WHAT The cardiovascular system delivers oxygen and HOW nutrients to the body tissues The heart pumps and carries away wastes blood throughout the body such as carbon dioxide in blood vessels. Blood flow via blood. requires both the pumping action of the heart and changes in blood pressure. WHY If the cardiovascular system cannot perform its functions, wastes build up in tissues. INSTRUCTORS Body organs fail to function properly, New Building Vocabulary and then, once oxygen becomes Coaching Activities for this depleted, they will die. chapter are assignable in hen most people hear the term cardio- only with the interstitial fluid in their immediate Wvascular system, they immediately think vicinity. Thus, some means of changing and of the heart. We have all felt our own “refreshing” these fluids is necessary to renew the heart “pound” from time to time when we are ner- nutrients and prevent pollution caused by vous. The crucial importance of the heart has been the buildup of wastes. Like a bustling factory, the recognized for ages. However, the cardiovascular body must have a transportation system to carry system is much more than just the heart, and its various “cargoes” back and forth. Instead of from a scientific and medical standpoint, it is roads, railway tracks, and subways, the body’s important to understand why this system is so vital delivery routes are its hollow blood vessels. to life. Most simply stated, the major function of the Night and day, minute after minute, our tril- cardiovascular system is transportation. Using lions of cells take up nutrients and excrete wastes. -

The Surgical Treatment of Constrictive Fibrous Endocarditis
The Surgical Treatment of Constrictive Fibrous Endocarditis CH. DUBOST, P. MAURICE, A. GERBAUX, E. BERTRAND, R. RULLIERE, F. VIAL, A. BARRILLON, C. PRIGENT, A. CARPENTIER, R. SOYER Constrictive fibrous endocarditis is a pathological entity described From the Department of Cardiovascular Surgery, by Lo6ffler in 1936. Its etiology is unknown. The clinical course Broussais Hospital, Paris, France is characterized by an evolution towards cardiac insufficiency leading rapidly to a fatal outcome. Modern paraclinical investi- gations are necessary to assess the diagnostic. Cardiac catheteriza- tion brings the proof of adiastole and angiocardiography re- Endocardiectomy was first performed by us in 19715-7 veals the shape of amputation of the ventricle with auriculo- and the patient was improved. Since then, four more cases ventricular regurgitation. The operative procedure consists of have been done, either right or left sided or combined resection of the ventricular fibrosis including the valves and endocardiectomy and there is no doubt that a better auriculo-ventricular valve replacement 'by ;a prosthetic valve. The disease affects both Caucasians and Negros. Our experience knowledge of the clinical aspects of the disease will includes 5 cases. The indications for operation and their results increase the number of cases referred for treatment. are discussed. Pathology C ONSTRICTIVE FIBROUS ENDOCARDITIS, first described The development of fibrous endocarditis is similar in by Loeffler6 in 1936, consists of endocardial fibrosis all cases: it involves the filling chambers of one or several millimeters in thickness which is potentially both ventricles including the papillary muscles and constrictive and limited to the ventricular cavities. chordae tendinae of the mitral and tricuspid valves The clinical course is characterized by an evolution which themselves are included in the process in the towards cardiac insufficiency which may be predomi- majority of cases.8 nantly right or left sided leading rapidly to a fatal outcome. -

THE HEART Study Objectives
THE HEART Study Objectives: You are responsible to understand the basic structure of the four chambered heart, the position and structure of the valves, and the path of blood flow through the heart. Structure--The Outer Layer pericardium: a specialized name for the celomic sac that surrounds the heart. fibrous pericardium: the most outer, fibrous layer of the pericardium. serous pericardium: there are two surfaces (layers) to this tissue a) parietal layer: the outer layer of the serosa surrounding the heart. Lines the fibrous pericardium, and the combination of the two layers is also called the parietal pericardium. b) visceral layer: the inner layer of the serosa surrounding and adhering to the heart. Also called the visceral pericardium or epicardium. pericardial cavity: the potential space between the parietal and visceral layers of the serous pericardium. The cavity is filled with serous fluid Structure--The Heart: Blood is delivered to the right atrium from the coronary sinus, inferior vena cava and superior vena cava Blood travels to the right ventricle from the right atrium via the right atrioventricular valve Blood travels from the left atrium to the left ventricle via the left atrioventricular valve Blood leaves the heart via the pulmonary valve to the pulmonary trunk, is delivered to the lungs for oxygenation and returns via the pulmonary veins to the left atrium a) crista terminalis: separates the anterior rough-walled and posterior smooth-walled portions of the right atrium. b) right auricle: small, conical, muscular, pouch-like appendage. c) musculi pectinate (pectinate muscles): internal muscular ridges that fan out anteriorly from the crista terminalis. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5]. -

Kumka's Response to Stecco's Fascial Nomenclature Editorial
Journal of Bodywork & Movement Therapies (2014) 18, 591e598 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/jbmt FASCIA SCIENCE AND CLINICAL APPLICATIONS: RESPONSE Kumka’s response to Stecco’s fascial nomenclature editorial Myroslava Kumka, MD, PhD* Canadian Memorial Chiropractic College, Department of Anatomy, 6100 Leslie Street, Toronto, ON M2H 3J1, Canada Received 12 May 2014; received in revised form 13 May 2014; accepted 26 June 2014 Why are there so many discussions? response to the direction of various strains and stimuli. (De Zordo et al., 2009) Embedded with a range of mechanore- The clinical importance of fasciae (involvement in patho- ceptors and free nerve endings, it appears fascia has a role in logical conditions, manipulation, treatment) makes the proprioception, muscle tonicity, and pain generation. fascial system a subject of investigation using techniques (Schleip et al., 2005) Pathology and injury of fascia could ranging from direct imaging and dissections to in vitro potentially lead to modification of the entire efficiency of cellular modeling and mathematical algorithms (Chaudhry the locomotor system (van der Wal and Pubmed Exact, 2009). et al., 2008; Langevin et al., 2007). Despite being a topic of growing interest worldwide, This tissue is important for all manual therapists as a controversies still exist regarding the official definition, pain generator and potentially treatable entity through soft terminology, classification and clinical significance of fascia tissue and joint manipulative techniques. (Day et al., 2009) (Langevin et al., 2009; Mirkin, 2008). It is also reportedly treated with therapeutic modalities Lack of consistent terminology has a negative effect on such as therapeutic ultrasound, microcurrent, low level international communication within and outside many laser, acupuncture, and extracorporeal shockwave therapy. -

Structure of the Heart 2121
8941d_c21.qxd 8/13/03 2:19 PM Page 249 mac 106 mac 106:Desktop Folder:211_sks: EXERCISE STRUCTURE OF THE HEART 2121 OBJECTIVES MATERIALS After completing this exercise, you should • human heart model be able to: • human torso or chart showing the pulmonary and • Describe the location and coverings of the heart systemic circulations and the three layers of the heart wall • colored pencils • Identify major features of cardiac muscle tissue on • preserved sheep heart (or other mammal heart) a prepared microscope slide • dissecting instruments, trays, disposable gloves, • Identify the major heart structures on models or 5-inch blade knife charts • compound microscope with cardiac muscle slide • Describe the flow of a drop of blood through the (for demonstration) pulmonary and systemic circulations, listing the vessels, chambers, and valves • Describe the changes that take place in the heart after birth • Identify the selected heart structures on a dissected sheep heart our heart beats without external stimulation and rests A. COVERINGS only between heart beats. The heart is a small double Ypump that simultaneously pumps blood to and from AND LAYERS body cells through the systemic circulation and to and from OF THE HEART the lungs through the pulmonary circulation. The heart is about the size of a fist and lies in the thoracic cavity. The base of the heart is the wide superior portion of the heart from which the great vessels emerge, and the apex of the heart is the inferior end pointing to the left. The heart is tilted at an angle so that its inferior surface lies against the diaphragm with two-thirds of the heart to the left side of the sternum.