The Interventricular Septum by E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

The Ventricles
Guest Editorial Evolution of the Ventricles Solomon Victor, FRCS, FRCP We studied the evolution of ventricles by macroscopic examination of the hearts of Vijaya M. Nayak, MS marine cartilaginous and bony fish, and by angiocardiography and gross examination of Raveen Rajasingh, MPhil the hearts of air-breathing freshwater fish, frogs, turtles, snakes, and crocodiles. A right-sided, thin-walled ventricular lumen is seen in the fish, frog, turtle, and snake. In fish, there is external symmetry of the ventricle, internal asymmetry, and a thick- walled left ventricle with a small inlet chamber. In animals such as frogs, turtles, and snakes, the left ventricle exists as a small-cavitied contractile sponge. The high pressure generated by this spongy left ventricle, the direction of the jet, the ventriculoarterial ori- entation, and the bulbar spiral valve in the frog help to separate the systemic and pul- monary circulations. In the crocodile, the right aorta is connected to the left ventricle, and there is a complete interventricular septum and an improved left ventricular lumen when compared with turtles and snakes. The heart is housed in a rigid pericardial cavity in the shark, possibly to protect it from changing underwater pressure. The pericardial cavity in various species permits move- ments of the heart-which vary depending on the ventriculoarterial orientation and need for the ventricle to generate torque or spin on the ejected blood- that favor run-off into the appropriate arteries and their branches. In the lower species, it is not clear whether the spongy myocardium contributes to myocardial oxygenation. In human beings, spongy myocardium constitutes a rare form of congenital heart disease. -

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis
Received: 27 August 2019 | Revised: 4 February 2020 | Accepted: 20 February 2020 DOI: 10.1111/ahe.12549 SPECIAL ISSUE Cardiogenesis with a focus on vasculogenesis and angiogenesis Katrin Borasch1 | Kenneth Richardson2 | Johanna Plendl1 1Department of Veterinary Medicine, Institute of Veterinary Anatomy, Freie Abstract University Berlin, Berlin, Germany The initial intraembryonic vasculogenesis occurs in the cardiogenic mesoderm. Here, 2 College of Veterinary Medicine, School a cell population of proendocardial cells detaches from the mesoderm that subse- of Veterinary and Life Sciences, Murdoch University, Murdoch, WA, Australia quently generates the single endocardial tube by forming vascular plexuses. In the course of embryogenesis, the endocardium retains vasculogenic, angiogenic and Correspondence Johanna Plendl, Department of Veterinary haematopoietic potential. The coronary blood vessels that sustain the rapidly ex- Medicine, Institute of Veterinary Anatomy, panding myocardium develop in the course of the formation of the cardiac loop by Freie University Berlin, Berlin, Germany. Email: [email protected] vasculogenesis and angiogenesis from progenitor cells of the proepicardial serosa at the venous pole of the heart as well as from the endocardium and endothelial cells of Funding information Freie Universität Berlin the sinus venosus. Prospective coronary endothelial cells and progenitor cells of the coronary blood vessel walls (smooth muscle cells, perivascular cells) originate from different cell populations that are in close spatial as well as regulatory connection with each other. Vasculo- and angiogenesis of the coronary blood vessels are for a large part regulated by the epicardium and epicardium-derived cells. Vasculogenic and angiogenic signalling pathways include the vascular endothelial growth factors, the angiopoietins and the fibroblast growth factors and their receptors. -

Endocardial Cushion and Myocardial Defects After Cardiac Myocyte-Specific Conditional Deletion of the Bone Morphogenetic Protein Receptor ALK3
Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3 Vinciane Gaussin*†, Tom Van de Putte‡, Yuji Mishina§, Mark C. Hanks¶, An Zwijsen‡, Danny Huylebroeck‡, Richard R. Behringerʈ, and Michael D. Schneider*,** *Center for Cardiovascular Development, Baylor College of Medicine, Houston, TX 77030; ‡Flanders Interuniversity Institute for Biotechnology (VIB07), K.U. Leuven, 3000 Leuven, Belgium; §National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709; ¶Procter and Gamble Pharmaceuticals Health Care Research Center, 8700 Mason Montgomery Road, Mason, OH 45040; and ʈUniversity of Texas–M. D. Anderson Cancer Center, Houston, TX 77030 Edited by Eric N. Olson, University of Texas Southwestern Medical Center, Dallas, TX, and approved December 31, 2001 (received for review July 26, 2001) Receptors for bone morphogenetic proteins (BMPs), members of velopment, whereas ALK6 is absent from the heart at mid- the transforming growth factor- (TGF) superfamily, are persis- gestation (17). The developing heart also expresses ALK2͞ tently expressed during cardiac development, yet mice lacking type ActRIA (5, 18), which can function as a type I BMP receptor II or type IA BMP receptors die at gastrulation and cannot be used with preference for BMP6 and -7 (19). ALK3, ALK2, and to assess potential later roles in creation of the heart. Here, we BMPR-II are each essential for gastrulation and mesoderm used a Cre͞lox system for cardiac myocyte-specific deletion of the formation (18, 20, 21); mice lacking just BMP4 also fail to type IA BMP receptor, ALK3. ALK3 was specifically required at progress, typically, beyond the egg cylinder stage (22). -

Ventricular Septal Defect (VSD)
Ventricular Septal Defect (VSD) Ventricular Septal Defect. Flow of blood through a normal heart. What is a Ventricular Septal Defect?Your pet has been diagnosed with a Ventricular Septal Defect (VSD). A VSD is a malformation of the wall (interventricular septum) between the two pumping chambers (ventricles) allowing an abnormal communication. A VSD is a type of congenital defect, which means it is present from birth. VSDs are classified based upon whether they are restrictive or non-restrictive. In order to understand how this disease may affect your dog, it is important to understand normal circulation in the heart. Blood drains from the body into the right collecting chamber (called “atrium”) where it passes through the tricuspid valve and into the right pumping chamber (called “ventricle”). From here, blood is pumped into the pulmonary artery and subsequently to the lungs where it picks up oxygen. The oxygenated blood then drains passively into the left atrium, through the mitral valve, and into the left ventricle. The left ventricle then pumps the blood through the aorta and back to the body. Restrictive VSD: A restrictive VSD is a smaller diameter VSD that provides resistance of blood flow. These are the most common VSDs that we diagnose in dogs and cats. Due to normally higher pressures in the left side of the heart compared to the right side of the heart, most have blood flow from left-to-right through the hole. The amount of blood shunted depends on size of the VSD and the pressure difference across the VSD. Therefore, restrictive VSDs are further classified based on whether they are “hemodynamically significant” or not. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Cardiac Development Cardiac Development
CARDIAC DEVELOPMENT CARDIAC DEVELOPMENT Diane E. Spicer, BS, PA(ASCP) University of Florida Dept. of Pediatric Cardiology Curator – Van Mierop Cardiac Archive This lecture is given with special thanks to Professor RH Anderson, my mentor and my friend. Without his spectacular research and images of both human and mouse embryos, this lecture would not have been possible. CARDIAC DEVELOPMENT CARDIAC DEVELOPMENT ♥ What’s new? ♥ “An understanding of the elementary facts of human and comparative embryology is essential to an intelligent grasp of the ontogenetic problems of congenital cardiac disease.” ♥ Maude Abbott “Atlas of Congenital Cardiac Disease” American Heart Association, New York, 1936 CARDIACCARDIAC DEVELOPMENTDEVELOPMENT ♥ Do we need to change? ♥ In the past, most theories of morphogenesis were based on fanciful interpretation of normal development ♥ We are now able to demonstrate the anatomic and molecular changes that take place during cardiac development ♥ This now permits us to base our inferences on evidence, rather than speculation CARDIACCARDIAC DEVELOPMENTDEVELOPMENT ♥ It used to be thought that all components of the postnatal heart were contained within the initial linear heart tube ♥ In reality, new material is added at the arterial and venous poles from the second heart field. The initial tube, derived from the first heart field, forms little more than the definitive left ventricle Mouse embryo – 9 somites – Myosin LC Growth at arterial pole Putative left ventricle Growth at venous pole Mouse embryo – E8.5 – 9 somites -

Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition
biomolecules Article Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition 1, 2, 1 1 Luca Sgarra y, Alessandro Santo Bortone y, Maria Assunta Potenza , Carmela Nacci , Maria Antonietta De Salvia 1, Tommaso Acquaviva 2, Emanuela De Cillis 2, Marco Matteo Ciccone 2, Massimo Grimaldi 3 and Monica Montagnani 1,* 1 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (L.S.); [email protected] (M.A.P.); [email protected] (C.N.); [email protected] (M.A.D.S.) 2 Department of Emergency and Organ Transplantation—Section of Cardiovascular Diseases, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (A.S.B.); [email protected] (T.A.); [email protected] (E.D.C.); [email protected] (M.M.C.) 3 General Hospital “F. Miulli” Acquaviva delle Fonti, 70021 Bari, Italy; fi[email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 28 March 2020; Accepted: 2 June 2020; Published: 4 June 2020 Abstract: We explored the significance of the L-Arginine/asymmetric dimethylarginine (L-Arg/ADMA) ratio as a biomarker of endothelial dysfunction in stroke patients. To this aim, we evaluated the correlation, in terms of severity, between the degree of endothelial dysfunction (by L-Arg/ADMA ratio), the methylene tetrahydrofolate reductase (MTHFR) genotype, and the interatrial septum (IAS) phenotype in subject with a history of stroke. Methods and Results: L-Arg, ADMA, and MTHFR genotypes were evaluated; the IAS phenotype was assessed by transesophageal echocardiography. -
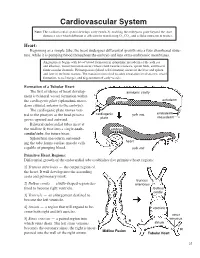
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle.