Endocardial Cushion and Myocardial Defects After Cardiac Myocyte-Specific Conditional Deletion of the Bone Morphogenetic Protein Receptor ALK3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ventricles
Guest Editorial Evolution of the Ventricles Solomon Victor, FRCS, FRCP We studied the evolution of ventricles by macroscopic examination of the hearts of Vijaya M. Nayak, MS marine cartilaginous and bony fish, and by angiocardiography and gross examination of Raveen Rajasingh, MPhil the hearts of air-breathing freshwater fish, frogs, turtles, snakes, and crocodiles. A right-sided, thin-walled ventricular lumen is seen in the fish, frog, turtle, and snake. In fish, there is external symmetry of the ventricle, internal asymmetry, and a thick- walled left ventricle with a small inlet chamber. In animals such as frogs, turtles, and snakes, the left ventricle exists as a small-cavitied contractile sponge. The high pressure generated by this spongy left ventricle, the direction of the jet, the ventriculoarterial ori- entation, and the bulbar spiral valve in the frog help to separate the systemic and pul- monary circulations. In the crocodile, the right aorta is connected to the left ventricle, and there is a complete interventricular septum and an improved left ventricular lumen when compared with turtles and snakes. The heart is housed in a rigid pericardial cavity in the shark, possibly to protect it from changing underwater pressure. The pericardial cavity in various species permits move- ments of the heart-which vary depending on the ventriculoarterial orientation and need for the ventricle to generate torque or spin on the ejected blood- that favor run-off into the appropriate arteries and their branches. In the lower species, it is not clear whether the spongy myocardium contributes to myocardial oxygenation. In human beings, spongy myocardium constitutes a rare form of congenital heart disease. -

Genetic and Flow Anomalies in Congenital Heart Disease
Published online: 2021-05-10 AIMS Genetics, 3(3): 157-166. DOI: 10.3934/genet.2016.3.157 Received: 01 July 2016 Accepted: 16 August 2016 Published: 23 August 2016 http://www.aimspress.com/journal/Genetics Review Genetic and flow anomalies in congenital heart disease Sandra Rugonyi* Department of Biomedical Engineering, Oregon Health & Science University, 3303 SW Bond Ave. M/C CH13B, Portland, OR 97239, USA * Correspondence: Email: [email protected]; Tel: +1-503-418-9310; Fax: +1-503-418-9311. Abstract: Congenital heart defects are the most common malformations in humans, affecting approximately 1% of newborn babies. While genetic causes of congenital heart disease have been studied, only less than 20% of human cases are clearly linked to genetic anomalies. The cause for the majority of the cases remains unknown. Heart formation is a finely orchestrated developmental process and slight disruptions of it can lead to severe malformations. Dysregulation of developmental processes leading to heart malformations are caused by genetic anomalies but also environmental factors including blood flow. Intra-cardiac blood flow dynamics plays a significant role regulating heart development and perturbations of blood flow lead to congenital heart defects in animal models. Defects that result from hemodynamic alterations recapitulate those observed in human babies, even those due to genetic anomalies and toxic teratogen exposure. Because important cardiac developmental events, such as valve formation and septation, occur under blood flow conditions while the heart is pumping, blood flow regulation of cardiac formation might be a critical factor determining cardiac phenotype. The contribution of flow to cardiac phenotype, however, is frequently ignored. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Ventricular Septal Defect (VSD)
Ventricular Septal Defect (VSD) Ventricular Septal Defect. Flow of blood through a normal heart. What is a Ventricular Septal Defect?Your pet has been diagnosed with a Ventricular Septal Defect (VSD). A VSD is a malformation of the wall (interventricular septum) between the two pumping chambers (ventricles) allowing an abnormal communication. A VSD is a type of congenital defect, which means it is present from birth. VSDs are classified based upon whether they are restrictive or non-restrictive. In order to understand how this disease may affect your dog, it is important to understand normal circulation in the heart. Blood drains from the body into the right collecting chamber (called “atrium”) where it passes through the tricuspid valve and into the right pumping chamber (called “ventricle”). From here, blood is pumped into the pulmonary artery and subsequently to the lungs where it picks up oxygen. The oxygenated blood then drains passively into the left atrium, through the mitral valve, and into the left ventricle. The left ventricle then pumps the blood through the aorta and back to the body. Restrictive VSD: A restrictive VSD is a smaller diameter VSD that provides resistance of blood flow. These are the most common VSDs that we diagnose in dogs and cats. Due to normally higher pressures in the left side of the heart compared to the right side of the heart, most have blood flow from left-to-right through the hole. The amount of blood shunted depends on size of the VSD and the pressure difference across the VSD. Therefore, restrictive VSDs are further classified based on whether they are “hemodynamically significant” or not. -
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the -

The Interventricular Septum by E
Thorax: first published as 10.1136/thx.12.4.304 on 1 December 1957. Downloaded from Thorax (1957), 12, 304. THE INTERVENTRICULAR SEPTUM BY E. W. T. MORRIS From the Anatomy Department, St. Thomas's Hospital Medical School, Londoni (RECEIVED FOR PUBLICATION JULY 26, 1957) It is difficult to find in the literature a clear and between the tips of its two horns where the concise account of the development and form of boundary is formed by the fused atrioventricular the interventricular septum. Moreover, some of cushion (A, in Fig. 4). This septum does not lie the accounts in the clinical literature are at vari- in one plane and the main part of its free border ance with that generally accepted by embryo- forms a spiral (Figs. 4 and 5). logists. For this reason and in view of the recent (2) While the muscular part is forming. changes technical advances in the surgery of the heart, it are taking place in the relative positions of the seems opportune to describe the development and bulbus cordis and the ventricles. Earlier the heart anatomy of the interventricular septum and to tube is flexed at the bulboventricular junction so correlate this knowledge as far as possible with that the bulbus cordis comes to lie ventrally and the sites of interventricular septal defects. to the right of the ventricle (Fig. 2). Their con- At an early stage the heart consists of the sinus tiguous walls form a septum-the bulboven- venosus, the common atrium, the common ven- tricular septum-around the lower free border tricle, and the bulbus cordis, serially arranged in of which the two cavities communicate (see Figs.copyright. -

Role of Nitric Oxide Synthase-3 in Heart Development
Differentiation 84 (2012) 54–61 Contents lists available at SciVerse ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff NOing the heart: Role of nitric oxide synthase-3 in heart development Yin Liu, Qingping Feng n Department of Physiology and Pharmacology, University of Western Ontario, London, Ontario, Canada N6A 5C1 article info abstract Available online 11 May 2012 Congenital heart disease is the most common birth defect in humans. Identifying factors that are critical to Keywords: embryonic heart development could further our understanding of the disease and lead to new strategies of Heart development its prevention and treatment. Nitric oxide synthase-3 (NOS3) or endothelial nitric oxide synthase (eNOS) is Nitric oxide known for many important biological functions including vasodilation, vascular homeostasis and Endothelial nitric oxide synthase angiogenesis. Over the past decade, studies from our lab and others have shown that NOS3 is required Congenital heart disease during heart development. More specifically, deficiency in NOS3 results in congenital septal defects, cardiac hypertrophy and postnatal heart failure. In addition, NOS3 is pivotal to the morphogenesis of major coronary arteries and myocardial capillary development. Interestingly, these effects of NOS3 are mediated through induction of transcription and growth factors that are crucial in the formation of coronary arteries. Finally, deficiency in NOS3 results in high incidences of bicuspid aortic valves, a disease in humans that often leads to complications with age including aortic valve stenosis or regurgitation, endocarditis, aortic aneurysm formation, and aortic dissection. In summary, these data suggest NOS3 plays a critical role in embryonic heart development and morphogenesis of coronary arteries and aortic valves. -

LAC.12 Embryology 2019-2020 Dr.Mahdi Alheety
LAC.12 Embryology 2019-2020 Dr.Mahdi ALheety Cardiovascular System Establishment of the Cardiogenic Field The vascular system appears in the middle of the third week, when the embryo is no longer able to satisfy its nutritional requirements by diffusion alone. Progenitor heart cells lie in the epiblast, immediately adjacent to the cranial end of the primitive streak. From there, they migrate through the streak and into the splanchnic layer of lateral plate mesoderm where they form a horseshoe-shaped cluster of cells called the primary heart field (PHF) cranial to the neural folds. As the progenitor heart cells migrate and form the PHF during days 16 to18, they are specified on both sides from lateral to medial to become the atria, left ventricle, and most of the right ventricle. Patterning of these cells occurs at the same time that laterality (left-right sidedness) is being established for the entire embryo and this process and the signaling pathway it is dependent upon is essential for normal heart development. The remainder of the heart, including part of the right ventricle and outflow tract (conus cordis and truncus arteriosus), is derived from the secondary heart field (SHF). This field of cells appears slightly later (days 20 to 21) than those in the PHF, resides in splanchnic mesoderm ventral to the posterior pharynx, and is responsible for lengthening the outflow tract. Cells in the SHF also exhibit laterality, such that those on the right side contribute to the left of the outflow tract region and those on the left contribute to the right. -
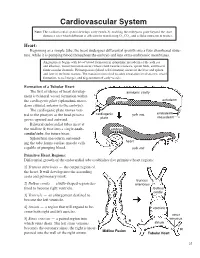
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Original Articles
Artigo Original %DVHV0RUIROyJLFDVSDUDR(VWXGRGR6HSWR,QWHUDWULDOQR)HWR Humano Morphological Basis for the Study of the Interatrial Septum in the Human Fetus Hugo Becker Amaral, Paulo Zielinsky, Aron Ferreira da Silveira, Ijoni Costabeber, Luiz Henrique Nicoloso, Olmiro Cezimbra de Souza Filho, Marcelo Salum, João Luiz Manica, Juliana Silveira Zanettini, Ane Micheli Costabeber 8QLGDGHGH&DUGLRORJLD)HWDOGR,QVWLWXWRGH&DUGLRORJLDGR5LR*UDQGHGR6XO'HSDUWDPHQWRGH0RUIRORJLDGR&HQWURGH&LrQFLDGD6D~GHGD Universidade Federal de Santa Maria – Porto Alegre, RS Resumo Objetivo: Descrever observações morfológicas sobre o septo interatrial em fetos normais, especialmente o forame oval e o septo primeiro, de forma a comparar a excursão do septo primeiro com o diâmetro do forame oval. Métodos: As medidas da excursão do septo primeiro (ESP) em direção ao átrio esquerdo (AE) e do diâmetro do forame oval (DFO) foram realizadas em corações de dez fetos humanos formolizados com 28 a 36 semanas. Os cortes histológicos foram feitos no FO, SP, septo segundo e nos AE e AD. Resultados: Os resultados da análise anatômica estão expressos em amplitude das medidas do DFO e da ESP: 3 fetos com idade gestacional (IG) presumida de 28 semanas, DFO (3,1-3,5 mm) e ESP (2,8-3,1 mm); 4 fetos com IG presumida de 34 semanas, DFO (3,3-3,5 mm) e ESP (4,0-5,0 mm); e 3 fetos com IG presumida de 36 semanas, DFO (3,3-4,5 mm) e ESP (6,0-9,0). Foram identificadas fibras musculares cardíacas no SP e no segundo. Conclusão: Pode-se sugerir que o SP apresenta caráter ativo devido às fibras musculares que o constituem, influenciando o fluxo sangüíneo através do FO, a mobilidade do SP e a sua excursão para o interior do AE. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5].