Lymphangiogenic Signaling in the Epicardium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic and Flow Anomalies in Congenital Heart Disease
Published online: 2021-05-10 AIMS Genetics, 3(3): 157-166. DOI: 10.3934/genet.2016.3.157 Received: 01 July 2016 Accepted: 16 August 2016 Published: 23 August 2016 http://www.aimspress.com/journal/Genetics Review Genetic and flow anomalies in congenital heart disease Sandra Rugonyi* Department of Biomedical Engineering, Oregon Health & Science University, 3303 SW Bond Ave. M/C CH13B, Portland, OR 97239, USA * Correspondence: Email: [email protected]; Tel: +1-503-418-9310; Fax: +1-503-418-9311. Abstract: Congenital heart defects are the most common malformations in humans, affecting approximately 1% of newborn babies. While genetic causes of congenital heart disease have been studied, only less than 20% of human cases are clearly linked to genetic anomalies. The cause for the majority of the cases remains unknown. Heart formation is a finely orchestrated developmental process and slight disruptions of it can lead to severe malformations. Dysregulation of developmental processes leading to heart malformations are caused by genetic anomalies but also environmental factors including blood flow. Intra-cardiac blood flow dynamics plays a significant role regulating heart development and perturbations of blood flow lead to congenital heart defects in animal models. Defects that result from hemodynamic alterations recapitulate those observed in human babies, even those due to genetic anomalies and toxic teratogen exposure. Because important cardiac developmental events, such as valve formation and septation, occur under blood flow conditions while the heart is pumping, blood flow regulation of cardiac formation might be a critical factor determining cardiac phenotype. The contribution of flow to cardiac phenotype, however, is frequently ignored. -

Endocardial Cushion and Myocardial Defects After Cardiac Myocyte-Specific Conditional Deletion of the Bone Morphogenetic Protein Receptor ALK3
Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3 Vinciane Gaussin*†, Tom Van de Putte‡, Yuji Mishina§, Mark C. Hanks¶, An Zwijsen‡, Danny Huylebroeck‡, Richard R. Behringerʈ, and Michael D. Schneider*,** *Center for Cardiovascular Development, Baylor College of Medicine, Houston, TX 77030; ‡Flanders Interuniversity Institute for Biotechnology (VIB07), K.U. Leuven, 3000 Leuven, Belgium; §National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709; ¶Procter and Gamble Pharmaceuticals Health Care Research Center, 8700 Mason Montgomery Road, Mason, OH 45040; and ʈUniversity of Texas–M. D. Anderson Cancer Center, Houston, TX 77030 Edited by Eric N. Olson, University of Texas Southwestern Medical Center, Dallas, TX, and approved December 31, 2001 (received for review July 26, 2001) Receptors for bone morphogenetic proteins (BMPs), members of velopment, whereas ALK6 is absent from the heart at mid- the transforming growth factor- (TGF) superfamily, are persis- gestation (17). The developing heart also expresses ALK2͞ tently expressed during cardiac development, yet mice lacking type ActRIA (5, 18), which can function as a type I BMP receptor II or type IA BMP receptors die at gastrulation and cannot be used with preference for BMP6 and -7 (19). ALK3, ALK2, and to assess potential later roles in creation of the heart. Here, we BMPR-II are each essential for gastrulation and mesoderm used a Cre͞lox system for cardiac myocyte-specific deletion of the formation (18, 20, 21); mice lacking just BMP4 also fail to type IA BMP receptor, ALK3. ALK3 was specifically required at progress, typically, beyond the egg cylinder stage (22). -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the -

Role of Nitric Oxide Synthase-3 in Heart Development
Differentiation 84 (2012) 54–61 Contents lists available at SciVerse ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff NOing the heart: Role of nitric oxide synthase-3 in heart development Yin Liu, Qingping Feng n Department of Physiology and Pharmacology, University of Western Ontario, London, Ontario, Canada N6A 5C1 article info abstract Available online 11 May 2012 Congenital heart disease is the most common birth defect in humans. Identifying factors that are critical to Keywords: embryonic heart development could further our understanding of the disease and lead to new strategies of Heart development its prevention and treatment. Nitric oxide synthase-3 (NOS3) or endothelial nitric oxide synthase (eNOS) is Nitric oxide known for many important biological functions including vasodilation, vascular homeostasis and Endothelial nitric oxide synthase angiogenesis. Over the past decade, studies from our lab and others have shown that NOS3 is required Congenital heart disease during heart development. More specifically, deficiency in NOS3 results in congenital septal defects, cardiac hypertrophy and postnatal heart failure. In addition, NOS3 is pivotal to the morphogenesis of major coronary arteries and myocardial capillary development. Interestingly, these effects of NOS3 are mediated through induction of transcription and growth factors that are crucial in the formation of coronary arteries. Finally, deficiency in NOS3 results in high incidences of bicuspid aortic valves, a disease in humans that often leads to complications with age including aortic valve stenosis or regurgitation, endocarditis, aortic aneurysm formation, and aortic dissection. In summary, these data suggest NOS3 plays a critical role in embryonic heart development and morphogenesis of coronary arteries and aortic valves. -

LAC.12 Embryology 2019-2020 Dr.Mahdi Alheety
LAC.12 Embryology 2019-2020 Dr.Mahdi ALheety Cardiovascular System Establishment of the Cardiogenic Field The vascular system appears in the middle of the third week, when the embryo is no longer able to satisfy its nutritional requirements by diffusion alone. Progenitor heart cells lie in the epiblast, immediately adjacent to the cranial end of the primitive streak. From there, they migrate through the streak and into the splanchnic layer of lateral plate mesoderm where they form a horseshoe-shaped cluster of cells called the primary heart field (PHF) cranial to the neural folds. As the progenitor heart cells migrate and form the PHF during days 16 to18, they are specified on both sides from lateral to medial to become the atria, left ventricle, and most of the right ventricle. Patterning of these cells occurs at the same time that laterality (left-right sidedness) is being established for the entire embryo and this process and the signaling pathway it is dependent upon is essential for normal heart development. The remainder of the heart, including part of the right ventricle and outflow tract (conus cordis and truncus arteriosus), is derived from the secondary heart field (SHF). This field of cells appears slightly later (days 20 to 21) than those in the PHF, resides in splanchnic mesoderm ventral to the posterior pharynx, and is responsible for lengthening the outflow tract. Cells in the SHF also exhibit laterality, such that those on the right side contribute to the left of the outflow tract region and those on the left contribute to the right. -
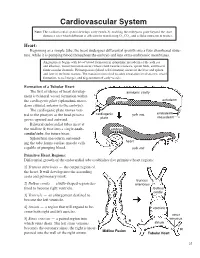
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Original Articles
Artigo Original %DVHV0RUIROyJLFDVSDUDR(VWXGRGR6HSWR,QWHUDWULDOQR)HWR Humano Morphological Basis for the Study of the Interatrial Septum in the Human Fetus Hugo Becker Amaral, Paulo Zielinsky, Aron Ferreira da Silveira, Ijoni Costabeber, Luiz Henrique Nicoloso, Olmiro Cezimbra de Souza Filho, Marcelo Salum, João Luiz Manica, Juliana Silveira Zanettini, Ane Micheli Costabeber 8QLGDGHGH&DUGLRORJLD)HWDOGR,QVWLWXWRGH&DUGLRORJLDGR5LR*UDQGHGR6XO'HSDUWDPHQWRGH0RUIRORJLDGR&HQWURGH&LrQFLDGD6D~GHGD Universidade Federal de Santa Maria – Porto Alegre, RS Resumo Objetivo: Descrever observações morfológicas sobre o septo interatrial em fetos normais, especialmente o forame oval e o septo primeiro, de forma a comparar a excursão do septo primeiro com o diâmetro do forame oval. Métodos: As medidas da excursão do septo primeiro (ESP) em direção ao átrio esquerdo (AE) e do diâmetro do forame oval (DFO) foram realizadas em corações de dez fetos humanos formolizados com 28 a 36 semanas. Os cortes histológicos foram feitos no FO, SP, septo segundo e nos AE e AD. Resultados: Os resultados da análise anatômica estão expressos em amplitude das medidas do DFO e da ESP: 3 fetos com idade gestacional (IG) presumida de 28 semanas, DFO (3,1-3,5 mm) e ESP (2,8-3,1 mm); 4 fetos com IG presumida de 34 semanas, DFO (3,3-3,5 mm) e ESP (4,0-5,0 mm); e 3 fetos com IG presumida de 36 semanas, DFO (3,3-4,5 mm) e ESP (6,0-9,0). Foram identificadas fibras musculares cardíacas no SP e no segundo. Conclusão: Pode-se sugerir que o SP apresenta caráter ativo devido às fibras musculares que o constituem, influenciando o fluxo sangüíneo através do FO, a mobilidade do SP e a sua excursão para o interior do AE. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5]. -

A Critical Role for the Epha3 Receptor Tyrosine Kinase in Heart Development ⁎ Lesley J
Developmental Biology 302 (2007) 66–79 www.elsevier.com/locate/ydbio A critical role for the EphA3 receptor tyrosine kinase in heart development ⁎ Lesley J. Stephen a, Amy L. Fawkes a, Adam Verhoeve a, Greg Lemke b, Arthur Brown a, a BioTherapeutics Research Group, The John P. Robarts Research Institute, and The University of Western Ontario, London, Ontario, Canada N6A 5K8 b Molecular Neurobiology Laboratory, The Salk Institute for Biological Studies, La Jolla, CA 92037, USA Received for publication 29 May 2006; revised 23 August 2006; accepted 24 August 2006 Available online 30 August 2006 Abstract Eph proteins are receptor tyrosine kinases that control changes in cell shape and migration during development. We now describe a critical role for EphA3 receptor signaling in heart development as revealed by the phenotype of EphA3 null mice. During heart development mesenchymal outgrowths, the atrioventricular endocardial cushions, form in the atrioventricular canal. This morphogenetic event requires endocardial cushion cells to undergo an epithelial to mesenchymal transformation (EMT), and results in the formation of the atrioventricular valves and membranous portions of the atrial and ventricular septa. We show that EphA3 knockouts have significant defects in the development of their atrial septa and atrioventricular endocardial cushions, and that these cardiac abnormalities lead to the death of approximately 75% of homozygous EphA3−/− mutants. We demonstrate that EphA3 and its ligand, ephrin-A1, are expressed in adjacent cells in the developing endocardial cushions. We further demonstrate that EphA3−/− atrioventricular endocardial cushions are hypoplastic compared to wildtype and that EphA3−/− endocardial cushion explants give rise to fewer migrating mesenchymal cells than wildtype explants. -

Complete Endocardial Cushion Defects in Pregnancy: a Case Report Xiangjuan Chen1, Biru Xiao1, Weiyu Yang2, Yunqin Chen1, Wenmiao Zhang1 and Haiyan Zhu1*
Chen et al. Journal of Medical Case Reports 2014, 8:91 JOURNAL OF MEDICAL http://www.jmedicalcasereports.com/content/8/1/91 CASE REPORTS CASE REPORT Open Access Complete endocardial cushion defects in pregnancy: a case report Xiangjuan Chen1, Biru Xiao1, Weiyu Yang2, Yunqin Chen1, Wenmiao Zhang1 and Haiyan Zhu1* Abstract Introduction: Complete endocardial cushion defect is a congenital heart disease characterized by a variable deficiency of the atrioventricular area in the developing heart. The mortality rate for an unrepaired endocardial cushion defect in pregnancy and the postpartum period is high. Case presentation: We present a rare case of a pregnant woman with complete endocardial cushion defect. A 20-year-old Chinese woman with unrepaired complete endocardial cushion defect delivered a premature male baby at 33 weeks and six days of pregnancy in our hospital. The baby had a normal human karyotype and a birth defect of hypospadias deformity. Our patient died from heart failure 10 minutes after delivery. She had severe pulmonary hypertension and suspected trisomy 21. Conclusion: Our experience further emphasizes the necessity of prenatal screening for congenital heart defects and of prompt surgical correction for endocardial cushion defects during infancy. Mortality for endocardial cushion defect during pregnancy and the postpartum period is high and women with complete endocardial cushion defect should avoid pregnancy, especially those women who cannot intellectually judge their risks. Keywords: Complete endocardial cushion defect, Eisenmenger’s syndrome, Pregnancy Introduction India [2]. The cause of an ECD remains unclear. Agopian A complete endocardial cushion defect (ECD) involves a et al. reported that maternal pregestational diabetes large primum atrial septal defect and an inlet ventricular and obesity are significantly associated with a non- septal defect of variable size. -

Septum Primum
LECTURE #1 1 EMBRYOLOGY 2 By the end of the lecture, you should be able to: 3 Describe the Formation, Site, Union, Division of the 5 heart tube. 6 Describe the formation and fate of the (Sinus 7 Venosus). Describe the formation of the interatrial and the 11 interventricular septae. 12 Describe the formation of the two atria and the two 15 ventricles. 16 Describe the partitioning of the Truncus arteriosus and formation of the aorta and pulmonary trunk. 17 List the most common cardiac anomalies. 2 EMBRYOLOGY • It’s the first functional organ to develop. • Origin: cardiogenic area of splanchnic mesoderm • The heart primordium can be seen at 18 days as an angioplastic cords which will be canalized to form the 2 heart tubes. • It begins cranial to the developing mouth & nervous system and ventral to the developing pericardial sac (as in A) , after the head fold completed the developing heart tubes lie in the ventral aspect of the embryo dorsal to the developing pericardial sac. (as in C) cranial Ventral Dorsa l • It begins to beat at 22 to 23 days. • Blood flow begins during the beginning of the fourth week. • can be visualized by Ultrasound Doppler 3 EMBRYOLOGY • the 2 heart tubes approach each other After lateral folding of the embryo and fuse to form a single endocardial heart tube within the pericardial sac. • The fusing occurs in a craniocaudal direction. • The heart tube grows faster than the pericardial sac, so it shows 5 alternate dilations separated by 4 constrictions: * Sinus venosus, the venous end * Truncus Arteriosus, the Arterial end * Common Ventricle.