P-Block Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5 Heavy Metals As Endocrine-Disrupting Chemicals
5 Heavy Metals as Endocrine-Disrupting Chemicals Cheryl A. Dyer, PHD CONTENTS 1 Introduction 2 Arsenic 3 Cadmium 4 Lead 5 Mercury 6 Uranium 7 Conclusions 1. INTRODUCTION Heavy metals are present in our environment as they formed during the earth’s birth. Their increased dispersal is a function of their usefulness during our growing dependence on industrial modification and manipulation of our environment (1,2). There is no consensus chemical definition of a heavy metal. Within the periodic table, they comprise a block of all the metals in Groups 3–16 that are in periods 4 and greater. These elements acquired the name heavy metals because they all have high densities, >5 g/cm3 (2). Their role as putative endocrine-disrupting chemicals is due to their chemistry and not their density. Their popular use in our industrial world is due to their physical, chemical, or in the case of uranium, radioactive properties. Because of the reactivity of heavy metals, small or trace amounts of elements such as iron, copper, manganese, and zinc are important in biologic processes, but at higher concentrations they often are toxic. Previous studies have demonstrated that some organic molecules, predominantly those containing phenolic or ring structures, may exhibit estrogenic mimicry through actions on the estrogen receptor. These xenoestrogens typically are non-steroidal organic chemicals released into the environment through agricultural spraying, indus- trial activities, urban waste and/or consumer products that include organochlorine pesticides, polychlorinated biphenyls, bisphenol A, phthalates, alkylphenols, and parabens (1). This definition of xenoestrogens needs to be extended, as recent investi- gations have yielded the paradoxical observation that heavy metals mimic the biologic From: Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice Edited by: A. -

Lanthanides & Actinides Notes
- 1 - LANTHANIDES & ACTINIDES NOTES General Background Mnemonics Lanthanides Lanthanide Chemistry Presents No Problems Since Everyone Goes To Doctor Heyes' Excruciatingly Thorough Yearly Lectures La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Although Theorists Prefer Unusual New Proofs Able Chemists Believe Careful Experiments Find More New Laws Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Principal Characteristics of the Rare Earth Elements 1. Occur together in nature, in minerals, e.g. monazite (a mixed rare earth phosphate). 2. Very similar chemical properties. Found combined with non-metals largely in the 3+ oxidation state, with little tendency to variable valence. 3. Small difference in solubility / complex formation etc. of M3+ are due to size effects. Traversing the series r(M3+) steadily decreases – the lanthanide contraction. Difficult to separate and differentiate, e.g. in 1911 James performed 15000 recrystallisations to get pure Tm(BrO3)3! f-Orbitals The Effective Electron Potential: • Large angular momentum for an f-orbital (l = 3). • Large centrifugal potential tends to keep the electron away from the nucleus. o Aufbau order. • Increased Z increases Coulombic attraction to a larger extent for smaller n due to a proportionately greater change in Zeff. o Reasserts Hydrogenic order. This can be viewed empirically as due to differing penetration effects. Radial Wavefunctions Pn,l2 for 4f, 5d, 6s in Ce 4f orbitals (and the atoms in general) steadily contract across the lanthanide series. Effective electron potential for the excited states of Ba {[Xe] 6s 4f} & La {[Xe] 6s 5d 4f} show a sudden change in the broadness & depth of the 4f "inner well". -
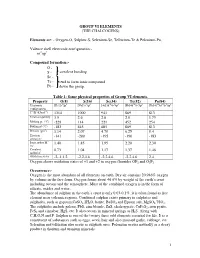
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -
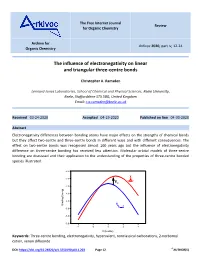
The Influence of Electronegativity on Linear and Triangular Three-Centre Bonds
The Free Internet Journal Review for Organic Chemistry Archive for Arkivoc 2020, part iv, 12-24 Organic Chemistry The influence of electronegativity on linear and triangular three-centre bonds Christopher A. Ramsden Lennard-Jones Laboratories, School of Chemical and Physical Sciences, Keele University, Keele, Staffordshire ST5 5BG, United Kingdom Email: [email protected] Received 03-24-2020 Accepted 04-19-2020 Published on line 04-30-2020 Abstract Electronegativity differences between bonding atoms have major effects on the strengths of chemical bonds but they affect two-centre and three-centre bonds in different ways and with different consequences. The effect on two-centre bonds was recognised almost 100 years ago but the influence of electronegativity difference on three-centre bonding has received less attention. Molecular orbital models of three-centre bonding are discussed and their application to the understanding of the properties of three-centre bonded species illustrated. -2.5 X -3.0 + Y Y Ea -3.5 -4.0 -4.5 X Binding Energy Binding + -5.0 Y Y -5.5 -6.0 -4 -2 0 2 4 h (b units) Keywords: Three-centre bonding, electronegativity, hypervalent, nonclassical carbocations, 2-norbornyl cation, xenon difluoride DOI: https://doi.org/10.24820/ark.5550190.p011.203 Page 12 ©AUTHOR(S) Arkivoc 2020, iv, 12-24 Ramsden, C. A. Table of Contents 1. Introduction 2. Linear Three-Centre Bonds (Hypervalent Bonds) (X-Y-X) X 3. Triangular Three-Centre Bonds (Y Y) 4. The Localised Bond Model of Two- and Three-Centre Bonds 5. Conclusions References -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
![Arxiv:2001.06745V2 [Nucl-Th] 6 Jun 2021 Osrit Nteeuto Fsaeo Eto-Ihncermat Nuclear [5–9]](https://docslib.b-cdn.net/cover/8390/arxiv-2001-06745v2-nucl-th-6-jun-2021-osrit-nteeuto-fsaeo-eto-ihncermat-nuclear-5-9-118390.webp)
Arxiv:2001.06745V2 [Nucl-Th] 6 Jun 2021 Osrit Nteeuto Fsaeo Eto-Ihncermat Nuclear [5–9]
Neutron drip line of Z =9 − 11 isotopic chains Rong An,1 Guo-Fang Shen,1 Shi-Sheng Zhang,1, ∗ and Li-Sheng Geng1, 2, 3, 4, † 1School of Physics, Beihang University, Beijing 100191, China 2Beijing Key Laboratory of Advanced Nuclear Materials and Physics, Beihang University, Beijing 100191, China 3Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Beihang University, Beijing100191, China 4School of Physics and Microelectronics, Zhengzhou University, Zhengzhou, Henan 450001, China A recent experimental breakthrough identified the last bound neutron-rich nuclei in fluorine and neon isotopes. Based on this finding, we perform a theoretical study of Z = 9, 10, 11, 12 isotopes in the relativistic mean field (RMF) model. The mean field parameters are assumed from the PK1 parameterization, and the pairing correlation is described by the particle number conservation BCS (FBCS) method recently formulated in the RMF model. We show that the FBCS approach plays an essential role in reproducing experimental results of fluorine and neon isotopes. Furthermore, we predict 39Na and 40Mg to be the last bound neutron-rich nuclei in sodium and magnesium isotopes. I. INTRODUCTION Properties of neutron-rich nuclei, in particular, the location of the neutron drip line, play an important role not only in understanding nuclear stability with respect to the isospin in hereto unexplored regions of the nuclear chart [1], but also in numerous related scientific issues of current interests. For instance, the r-process nucleosynthesis of heavy elements in stellar evolution crucially depends on the values of beta decay rates and neutron capture cross sections in neutron-rich nuclei that do not exist in terrestrial conditions [2–4]. -

Amarsinha D. Nikam Vital Force Is Oxygen Extrait Du Livre Vital Force Is Oxygen De Amarsinha D
Amarsinha D. Nikam Vital Force is Oxygen Extrait du livre Vital Force is Oxygen de Amarsinha D. Nikam Éditeur : B. Jain http://www.editions-narayana.fr/b9138 Sur notre librairie en ligne vous trouverez un grand choix de livres d'homéopathie en français, anglais et allemand. Reproduction des extraits strictement interdite. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern, Allemagne Tel. +33 9 7044 6488 Email [email protected] http://www.editions-narayana.fr Oxygen Oxygen is derived from the Greek word, where oxys means acid, literally sharp from the taste of acids and genes means producer, literally begetter. It is the element with atomic number 8 and represented by the symbol 'O'. It is a highly reactive non-metallic period 2 element that readily forms compounds (notably oxides) with almost all other elements. Oxygen is the third most abundant element in the universe by mass after hydrogen and helium. Diatomic oxygen gas constitutes 21% of the volume of air. Water is the most familiar oxygen compound. OXYGEN HISTORY Oxygen makes up 21% of the atmosphere we breathe, but it was not discovered as a separate gas until the late i8th century. Oxygen was independently discovered by Carl Wilhelm Scheele, in Uppsala in 1773 or earlier and by Joseph Priestley in Wiltshire, in 1774. The name oxygen was coined in 1777 by Antoine Lavoisier. Although oxygen plays a life-supporting role, it took Narayana Verlag, 79400 Kandern Tel.: 0049 7626 974 970 0 Excerpt from Dr. Amarsinha D.Nikam: Vital Force is Oxygen about 150 years for the gas to be used in a proper manner for patients. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

1 9729/01/TJC Prelim/2018 [Turn Over
1 CHEMISTRY 9729/01 Paper 1 Multiple Choice 15th September 2018 1 hour Additional materials: Multiple Choice Answer Sheet Data Booklet READ THESE INSTRUCTIONS FIRST Write in soft pencil. Do not use staples, paper clips, glue or correction fluid. Write your name and Civics Group Write and shade your index number There are thirty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C and D. Choose the one you consider correct and record your choice in soft pencil on the separate Answer Sheet. Read the instructions on the Answer Sheet very carefully. Each correct answer will score one mark. A mark will not be deducted for a wrong answer. Any rough working should be done in this booklet. The use of an approved scientific calculator is expected, where appropriate. This document consists of 13 printed pages. 9729/01/TJC Prelim/2018 [Turn Over 2 Section A For each question there are four possible answers, A, B, C and D. Choose the one you consider correct and record your choice in soft pencil on the separate Answer Sheet (OMS). 1 Some isotopes are unstable and undergo nuclear (radioactive) reactions. In one type of reaction, an unstable nucleus assimilates an electron from an inner orbital of its electron cloud. The net effect is the conversion of a proton and an electron into a neutron. Which of the following describes this type of reaction? A 11C 12C B 111I 111Te C 76Br 75Br D 76Kr 75Br 2 Sodium hydrogencarbonate can be prepared from sodium sulfate by a three-step process: Na2SO4(s) + 4C(s) -

New Technologies for 211At Targeted Α-Therapy Research Using 211Rn and 209At
New technologies for 211At targeted α-therapy research using 211Rn and 209At Jason Raymond Crawford BSc, University of British Columbia, 2007 MSc, University of Victoria, 2010 A dissertation submitted in partial fulfilment of the requirements for the degree of Doctorate of Philosophy in the Department of Physics and Astronomy c Jason Raymond Crawford, 2016 University of Victoria All rights reserved. This dissertation may not be reproduced in whole or in part by photocopy or other means, without the permission of the author. ii New technologies for 211At targeted α-therapy research using 211Rn and 209At by Jason Raymond Crawford BSc, University of British Columbia, 2007 MSc, University of Victoria, 2010 Supervisory Committee Dr. Thomas J Ruth, Co-Supervisor Department of Physics and Astronomy Dr. Andrew Jirasek, Co-Supervisor Department of Physics and Astronomy Dr. Wayne Beckham, Committee Member Department of Physics and Astronomy Dr. Dean Karlen, Committee Member Department of Physics and Astronomy Dr. Julian Lum, Outside Member Department of Biochemistry & Microbiology iii Abstract The most promising applications for targeted α-therapy with astatine-211 (211At) in- clude treatments of disseminated microscopic disease, the major medical problem for cancer treatment. The primary advantages of targeted α-therapy with 211At are that the α-particle radiation is densely ionizing, translating to high relative biological effectiveness (RBE), and short-range, minimizing damage to surrounding healthy tissues. In addition, theranostic imaging with 123I surrogates has shown promise for developing new therapies with 211At and translating them to the clinic. Currently, Canada does not have a way of producing 211At by conventional methods because it lacks α-particle accelerators with necessary beam energy and intensity. -

Environment and Ecology BHM 403 Techno India
Environment and Ecology BHM 403 Techno India ENVIRONMENT AND ECOLOGY BHM 403 (2008 -2017) PREPARED BY ANIS CHATTOPADHYAY ASST. PROFESSOR TECHNO INDIA EM-4/1, SECTOR –V, SALT LAKE KOLKATA -700091 1 Environment and Ecology BHM 403 Techno India 2008 GROUP – A ( Multiple Choice Type Questions ) 1. Choose correct answer from the given alternatives in each of the following questions : 10x1 = 10 i) Environmental Studies involve studies of a) evolution of life b) all aspects of human environment c) nitrogen cycle d) oxygen cycle. b ii) "Itai itai' disease is caused by a) Zinc b) Cadmium c) Mercury d) Iron. b iii) El Nino starts from a) Mediterranean coast b) Chinese coast c) South American cost d) Indian cost. C iv) The Greenhouse effect is due to a) Carbon dioxide, water vapour, methane and chlorofluorocarbons b) Nitrogen oxide c) Sulphur oxide d) Carbon monoxide. A v) The Ganga pollution is due to dumping of a) domestic and industrial sewages b) waste from forest c) food waste d) hospital waste. A vi) Biotic factor of ecosystem is a) Solar energy b) Temperature c) Soil d) Plants and animals. D vii) Medha Patkar is involved in a) Chipko movement b) Silent Valley movement c) Narmada Bachao movement d) none of these. C viii) The "Kyoto Protocol" is related with a) air pollution b) noise pollution c) water pollution d) none of these. A ix) BOD stands as a) Biochemical Oxygen Demand b) Biological Oxygen Demand c) Biggest Oxygen Demand d) Blown Out Dose. B x) The protective shield for life on earth is a) Carbon dioxide b) Ozone c) Oxygen d) Hydrogen.