Masterarbeit / Master's Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Araneae (Spider) Photos
Araneae (Spider) Photos Araneae (Spiders) About Information on: Spider Photos of Links to WWW Spiders Spiders of North America Relationships Spider Groups Spider Resources -- An Identification Manual About Spiders As in the other arachnid orders, appendage specialization is very important in the evolution of spiders. In spiders the five pairs of appendages of the prosoma (one of the two main body sections) that follow the chelicerae are the pedipalps followed by four pairs of walking legs. The pedipalps are modified to serve as mating organs by mature male spiders. These modifications are often very complicated and differences in their structure are important characteristics used by araneologists in the classification of spiders. Pedipalps in female spiders are structurally much simpler and are used for sensing, manipulating food and sometimes in locomotion. It is relatively easy to tell mature or nearly mature males from female spiders (at least in most groups) by looking at the pedipalps -- in females they look like functional but small legs while in males the ends tend to be enlarged, often greatly so. In young spiders these differences are not evident. There are also appendages on the opisthosoma (the rear body section, the one with no walking legs) the best known being the spinnerets. In the first spiders there were four pairs of spinnerets. Living spiders may have four e.g., (liphistiomorph spiders) or three pairs (e.g., mygalomorph and ecribellate araneomorphs) or three paris of spinnerets and a silk spinning plate called a cribellum (the earliest and many extant araneomorph spiders). Spinnerets' history as appendages is suggested in part by their being projections away from the opisthosoma and the fact that they may retain muscles for movement Much of the success of spiders traces directly to their extensive use of silk and poison. -

The Aromatic Head Group of Spider Toxin Polyamines Influences
toxins Article The Aromatic Head Group of Spider Toxin Polyamines Influences Toxicity to Cancer Cells David Wilson 1, Glen M. Boyle 2 ID , Lachlan McIntyre 3, Matthew J. Nolan 1 ID , Peter G. Parsons 2 ID , Jennifer J. Smith 4, Leon Tribolet 1, Alex Loukas 1, Michael J. Liddell 3, Lachlan D. Rash 4,5 ID and Norelle L. Daly 1,* 1 Centre for Biodiscovery and Molecular Development of Therapeutics, AITHM, James Cook University, Cairns, QLD 4878, Australia; [email protected] (D.W.); [email protected] (M.J.N.); [email protected] (L.T.); [email protected] (A.L.) 2 QIMR Berghofer Medical Research Institute, Herston, QLD 4006, Australia; [email protected] (G.M.B.); [email protected] (P.G.P.) 3 Centre for Tropical Environmental and Sustainable Sciences, James Cook University, Cairns, QLD 4878, Australia; [email protected] (L.M.); [email protected] (M.J.L.) 4 Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia; [email protected] (J.J.S.); [email protected] (L.D.R.) 5 School of Biomedical Sciences, University of Queensland, Brisbane, QLD 4072, Australia * Correspondence: [email protected]; Tel.: +61-7-4232-1815 Academic Editor: Hang Fai (Henry) Kwok Received: 5 September 2017; Accepted: 23 October 2017; Published: 27 October 2017 Abstract: Spider venoms constitute incredibly diverse libraries of compounds, many of which are involved in prey capture and defence. Polyamines are often prevalent in the venom and target ionotropic glutamate receptors. -
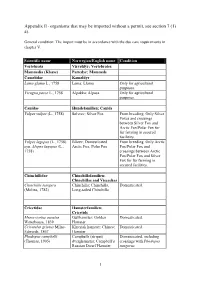
Organisms That May Be Imported Without a Permit, See Section 7 (1) A)
Appendix II - organisms that may be imported without a permit, see section 7 (1) a). General condition: The import must be in accordance with the due care requirements in chapter V. Scientific name Norwegian/English name Condition Vertebrata Virveldyr; Vertebrates Mammalia (Klasse) Pattedyr; Mammals Camelidae Kameldyr Lama glama L., 1758 Lama; Llama Only for agricultural purposes. Vicugna pacos L., 1758 Alpakka; Alpaca Only for agricultural purposes. Canidae Hundefamilien; Canids Vulpes vulpes (L., 1758) Sølvrev; Silver Fox From breeding. Only Silver Foxes and crossings between Silver Fox and Arctic Fox/Polar Fox for fur famring in secured facilities. Vulpes lagopus (L., 1758) Blårev; Domesticated From breeding. Only Arctic syn. Alopex lagopus (L., Arctic Fox, Polar Fox Fox/Polar Fox and 1758) crossings between Arctic Fox/Polar Fox and Silver Fox for fur farming in secured facilities. Chinchillidae Chinchillafamilien; Chinchillas and Viscachas Chinchilla lanigera Chinchilla; Chinchilla, Domesticated. (Molina, 1782) Long-tailed Chinchilla Cricetidae Hamsterfamilien; Cricetids Mesocricetus auratus Gullhamster; Golden Domesticated. Waterhouse, 1839 Hamster Cricetulus griseus Milne- Kinesisk hamster; Chinese Domesticated. Edwards, 1867 Hamster Phodopus campbelli Campbells (stripet) Domesticated, including (Thomas, 1905) dverghamster; Campbell’s crossings with Phodopus Russian Dwarf Hamster sungorus. 1 Appendix II - organisms that may be imported without a permit, see section 7 (1) a). General condition: The import must be in accordance with the due care requirements in chapter V. Scientific name Norwegian/English name Condition Phodopus sungorus (Pallas, Russisk (sibirsk) Domesticated, including 1773) dverghamster; Siberian crossings with Phodopus Hamster, Djungarian campbelli. Hamster Phodopus roborovski Roborovski dverghamster; Domesticated. (Satunin, 1903) Roborovski Hamster Caviidae Marsvinfamilien; Guinea Pigs Cavia porcellus (L., 1758) Marsvin; Guinea Pig Domesticated. -

Scwds Briefs
SCWDS BRIEFS A Quarterly Newsletter from the Southeastern Cooperative Wildlife Disease Study College of Veterinary Medicine The University of Georgia Phone (706) 542 - 1741 Athens, Georgia 30602 FAX (706) 542-5865 Volume 36 July 2020 Number 2 Working Together: The 40th Anniversary of the implications such as increasing host density around National Hemorrhagic Disease Survey water sources and wetland areas where Culicoides vectors breed. In the last two issues of SCWDS BRIEFS, we provided examples of how data from this long-term collaborative During 2007 and 2012, widespread HD outbreaks survey has been used to document the northern occurred in the eastern and midwestern United States, expansion of hemorrhagic disease (HD) in North and there was a spatial correlation between areas America and how such data can provide a map of HD affected by severe drought and areas where HD risk across broad landscapes. Understanding why the occurred. Previous research also has identified drought range of HD is expanding or why we observe these like conditions, such as high temperatures and low spatial patterns, however, is an ongoing challenge and precipitation, as risk factors for HD. To further our overall goal is not only to document HD occurrence investigate this potential relationship, we focused on 23 but to understand the mechanisms that drive the states in the eastern United States that have reported observed spatial and temporal patterns. In part three of HD related mortality during the period 2000-2014. this series we provide a recent example of how the data These included states where HD has historically from the HD survey have been used to investigate some occurred since 1980 and states such as Michigan, of the mechanisms that drive regional HD outbreaks. -

November 14, 1899
824 THB SWR~TABYON ADDITIONS m m~MBNAG~RIID. [Nov. 14, B. BRANCHED. a. Branching in one plane. Short, very slender, sparingly branched ; covered with spines. Antipathella, racilis (Gray). Macleim Spine8 on the ultimate branchlets only ; stom smoo$. Leiopatlaes expama J. Y. J. Madeira. No spines ; stem and branches wrinkled and punctured. 8auagli.a lamarcki (Hairne). Madei a, Mediterranean. h. Branching on all sides, buahy. Bpiues only on the ultimate branohlets. Leiopathcs glabewima M.-Edw. Ma- deira ; Mediterranean ; w. Indies. Spines sbort, triangiilar, upright, branches arranged in a corymbose manner. Antipathes furcata Gray. Madeira. Spines elongate, directed forwwds... Aphaiiipnthes wollastoni Brook. Ma- deira ; Selvngens. Var. pilosa with hairs on the stem. November 14, 1899. Dr. A. GBNTHEB,F.R.S., V.P., in the Chair. The Secretary read the following reports on the additions made to the Society’s Menagerie during the months of June, July, August, September, and October, 1899 :- The total number of registered additions to the Society’s Mena- gene during the month of .June was 164, of which 79 were by presentation, 18 by birth, 40 by purchase, 1 was received in exchange and 26 on deposit. The total number of departures during the same period, by death and removals, was 78. Among the additions special attention may be called to a male Sitatunga, or Speke’s Antelope (Tmgelaphus spekii), from the district of Lake Ngami, received from Mr. Oecil J. Rhodes, F.Z.S., in exchange for a female hybrid between Tragelaphus gratus d and T. spelcii 9 (born in the Menagerie on Feb. 12, l896), which was despatched to Mr. -
HEMOLYMPH CYTOLOGY, CELL COUNT, and ELECTROLYTE REFERENCE VALUES in CAMEROON RED TARANTULA (Hysterocrates Gigas)
HEMOLYMPH CYTOLOGY, CELL COUNT, AND ELECTROLYTE REFERENCE VALUES IN CAMEROON RED TARANTULA (Hysterocrates gigas) Cédric B. Larouche, DVM, IPSAV,1,2 Janet Beeler-Marfisi, BA, DVM, DVSc, Dipl ACVP,1 Lydia Attard, MSc,2 Nicole Nemeth, DVM, PhD, Dipl ACVP, 1 and Hugues Beaufrère, Dr.Med.Vet., PhD, Dipl ACZM, Dipl ECZM (Avian), Dipl ABVP (Avian)1 1Ontario Veterinary College, University of Guelph, Guelph, ON, N1G 2W1 Canada; 2Toronto Zoo, Toronto, ON, M1B 5K7 Canada Abstract: Tarantulas of the family Theraphosidae are commonly kept as pets or display animals in zoological institutions. Over the last decade, requests for arachnid medicine have increased, but the range of diagnostic tests is limited (Pizzi, 2011). Hemolymph analysis could be an important tool to evaluate arachnid health. Ninety-three mature Cameroon red tarantulas (Hysterocrates gigas) from the Toronto zoo were used to collect 0.35 ml of hemolymph from the dorsal opisthosoma. Hemocytes were characterized and reference intervals were established for absolute and differential hemocyte counts and electrolyte values (Scherman, 1981; Friedrichs et al, 2012; Soares, et al, 2013; Bednaski et al, 2015). These results help support a more quantitative assessment and thus a better understanding of arachnid health. Objectives: 1. To characterize the circulating hemocytes in the Cameroon red tarantula. 2. To provide reference intervals for hemocyte absolute and differential counts, as well as for hemolymph electrolyte values in the Cameroon red tarantula. Conclusion: Hemocyte counts for the Cameroon red tarantula indicate that plasmatocytes are predominant; granulocytes are common while cyanocytes and prohemocytes are rare. The hemolymph electrolyte values are comparable to other tarantula species but differ from dog and cat blood such that tarantulas have higher sodium, chloride, and ionized calcium, while having lower potassium and glucose levels. -

Cell-Based Reporter Release Assay to Determine the Activity of Calcium-Dependent Neurotoxins and Neuroactive Pharmaceuticals
toxins Article Cell-Based Reporter Release Assay to Determine the Activity of Calcium-Dependent Neurotoxins and Neuroactive Pharmaceuticals Andrea Pathe-Neuschäfer-Rube, Frank Neuschäfer-Rube * and Gerhard P. Püschel Department of Nutritional Biochemistry, Institute of Nutritional Science, University of Potsdam, 14469 Potsdam, Germany; [email protected] (A.P.-N.-R.); [email protected] (G.P.P.) * Correspondence: [email protected]; Tel.: +49-33200-88-569 Abstract: The suitability of a newly developed cell-based functional assay was tested for the detection of the activity of a range of neurotoxins and neuroactive pharmaceuticals which act by stimulation or inhibition of calcium-dependent neurotransmitter release. In this functional assay, a reporter enzyme is released concomitantly with the neurotransmitter from neurosecretory vesicles. The current study showed that the release of a luciferase from a differentiated human neuroblastoma-based reporter cell line (SIMA-hPOMC1-26-GLuc cells) can be stimulated by a carbachol-mediated activation of the Gq-coupled muscarinic-acetylcholine receptor and by the Ca2+-channel forming spider toxin α-latrotoxin. Carbachol-stimulated luciferase release was completely inhibited by the muscarinic acetylcholine receptor antagonist atropine and α-latrotoxin-mediated release by the Ca2+-chelator EGTA, demonstrating the specificity of luciferase-release stimulation. SIMA-hPOMC1-26-GLuc cells express mainly L- and N-type and to a lesser extent T-type VGCC on the mRNA and protein level. In accordance with the expression profile a depolarization-stimulated luciferase release by a high K+-buffer was effectively and dose-dependently inhibited by L-type VGCC inhibitors and to a lesser + Citation: Pathe-Neuschäfer-Rube, A.; extent by N-type and T-type inhibitors. -

UG ETD Template
Exploring genome size diversity in arachnid taxa by Haley Lauren Yorke A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Master of Science in Integrative Biology Guelph, Ontario, Canada © Haley L. Yorke, January, 2020 ABSTRACT EXPLORING GENOME SIZE DIVERSITY IN ARACHNID TAXA Haley L. Yorke Advisor(s): University of Guelph, 2019 Dr. T. Ryan Gregory This study investigates the general ranges and patterns of arachnid genome size diversity within and among major arachnid groups, with a focus on tarantulas (Theraphosidae) and scorpions (Scorpiones), and an examination of how genome size relates to body size, growth rate, longevity, and geographical latitude. I produced genome size measurements for 151 new species, including 108 tarantulas (Theraphosidae), 20 scorpions (Scorpiones), 17 whip-spiders (Amblypygi), 1 vinegaroon (Thelyphonida), and 5 non-arachnid relatives – centipedes (Chilopoda). I also developed a new methodology for non-lethal sampling of arachnid species that is inexpensive and portable, which will hopefully improve access to live specimens in zoological or hobbyist collections. I found that arachnid genome size is positively correlated with longevity, body size and growth rate. Despite this general trend, genome size was found to negatively correlate with longevity and growth rate in tarantulas (Theraphosidae). Tarantula (Theraphosidae) genome size was correlated negatively with latitude. iii ACKNOWLEDGEMENTS This study would not have been possible without access to specimens from the collections of both Amanda Gollaway and Martin Gamache of Tarantula Canada, and also of Patrik de los Reyes – thank you for trusting me with your valuable and much- loved animals, for welcoming me into your homes and places of business, and for your expert opinions on arachnid husbandry and biology. -

Therapeutic Potential of Venom Peptides
REVIEWS THERAPEUTIC POTENTIAL OF VENOM PEPTIDES Richard J. Lewis*‡ and Maria L. Garcia§ Venomous animals have evolved a vast array of peptide toxins for prey capture and defence. These peptides are directed against a wide variety of pharmacological targets, making them an invaluable source of ligands for studying the properties of these targets in different experimental paradigms. A number of these peptides have been used in vivo for proof-of-concept studies, with several having undergone preclinical or clinical development for the treatment of pain, diabetes, multiple sclerosis and cardiovascular diseases. Here we survey the pharmacology of venom peptides and assess their therapeutic prospects. Toxins have evolved in plants, animals and microbes well suited to addressing the crucial issues of potency DINOFLAGELLATES Unicellular algae that produce multiple times as part of defensive and/or prey capture and stability (FIG. 2).It is this evolved biodiversity that a range of toxins that enter the strategies. Non-peptide toxins are typically orally active makes venom peptides a unique source of leads and food chain during blooms. and include many food-borne toxins of DINOFLAGELLATE structural templates from which new therapeutic origin, such as the ciguatoxins and brevetoxins which agents might be developed. ENVENOMATION APPARATUS 1 Highly specialized glands activate voltage-sensitive sodium channels , the diar- In this review, peptides found in the venom of cone secreting peptides injected via rhoetic shellfish poisons which inhibit protein phos- snails, spiders, scorpions, snakes, the Gila monster lizard hollow teeth, stings, harpoons phatases2,3,and the paralytic shellfish poisons (saxitoxins) and sea anemone (FIG. 1) are discussed in relation to their and nematocyst tubules. -

Titas Petruša 6 Kursas, 21 Grupė BESIVYSTANČIŲ JUTIMINIŲ
LIETUVOS SVEIKATOS MOKSLŲ UNIVERSITETAS MEDICINOS AKADEMIJA MEDICINOS FAKULTETAS FIZIKOS, MATEMATIKOS IR BIOFIZIKOS KATEDRA Titas Petruša 6 kursas, 21 grupė BESIVYSTANČIŲ JUTIMINIŲ NEURONŲ ELEKTRINĖS SAVYBĖS Baigiamasis magistro darbas (Vientisųjų studijų programa- medicina) Darbo vadovas: Doc. Artūras Grigaliūnas Kaunas, 2020 1 Turinys 1. SANTRAUKA ..................................................................................................................................................... 4 2. SUMMARY ......................................................................................................................................................... 6 3. PADĖKA ............................................................................................................................................................. 8 4. INTERESŲ KONFLIKTAS ................................................................................................................................ 8 5. ETIKOS KOMITETO LEIDIMAS .................................................................................................................... 8 6. SANTRUMPOS ................................................................................................................................................... 9 7. SĄVOKOS ......................................................................................................................................................... 10 8. ĮVADAS ............................................................................................................................................................ -
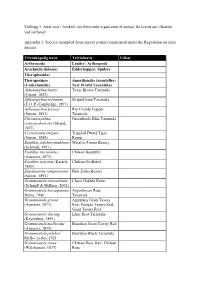
List of Species Exempted from Import Permit Requirement Pdf, 133.2
Vedlegg 1. Arter som i forskrift om fremmede organismer er unntatt fra kravet om tillatelse ved innførsel: Appendix 1. Species exempted from import permit requirement under the Regulation on alien species: Vitenskapelig navn Trivialnavn Vilkår Arthropoda Leddyr; Arthropods Arachnida (Klasse) Edderkopper; Spiders Theraphosidae Theraposinae Amerikanske taranteller; (Underfamilie) New World Tarantulas Aphonopelma hentzi Texas Brown Tarantula (Girard, 1852) Aphonopelma seemanni Striped-knee Tarantula (F.O. P.-Cambridge, 1897) Aphonopelma texense Rio Grande Copper (Simon, 1891) Tarantula Chromatopelma Greenbottle Blue Tarantula cyaneopubescens (Strand, 1907) Cyriocosmus elegans Trinidad Dwarf Tiger (Simon, 1889) Rump Euathlus pulcherrimaklaasi Metallic Femur Beauty (Schmidt, 1991) Euathlus truculentus Chilean Beautiful (Ausserer, 1875) Euathlus vulpinus (Karsch, Chilean Ocellated 1880) Eupalaestrus campestratus Pink Zebra Beauty (Simon, 1891) Grammostola aureostriata Chaco Golden Knee (Schmidt & Bullmer, 2001) Grammostola burzaquensis Argentinean Rose Ibarra, 1946 Tarantula Grammostola grossa Argentina Giant Tawny (Ausserer, 1871) Red, Pampas Tawny Red, Giant Tawny Red Grammostola iheringi Entre Rios Tarantula (Keyserling, 1891) Grammostola mollicoma Brazilian Giant Tawny Red (Ausserer, 1875) Grammostola pulchra Brazilian Black Tarantula Mello- Leitao, 1921 Grammostola rosea Chilean Rose Hair, Chilean (Walckenaer, 1837) Rose Vitenskapelig navn Trivialnavn Vilkår Lasiodora difficilis Mello- Fiery Redrump Leitao, 1921 Lasiodora klugi (C.L. Baja -
Appendix II – Organisms That Can Be Imported Without a Permit, See Section 7 (1) A)
Appendix II – organisms that can be imported without a permit, see section 7 (1) a) General condition: The import shall be carried out in accordance with the due care requirements set out in Chapter V and the rest of the Regulations, as well as other applicable law, including the Regulations of 15 November 2002 No. 1276 for the implementation of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) of 3 March 1973. Scientific name Norwegian/English name Condition Vertebrata Virveldyr; Vertebrates Mammalia (Klasse) Pattedyr; Mammals Camelidae Kameldyr Lama glama L., 1758 Lama; Llama Only for agricultural purposes. Vicugna pacos L., 1758 Alpakka; Alpaca Only for agricultural purposes. Canidae Hundefamilien; Canids Vulpes vulpes (L., 1758) Sølvrev; Silver Fox From breeding. Only Silver Foxes and crossings between Silver Fox and Arctic Fox/Polar Fox for fur farming when the keeping is carried out in accordance with the Regulations 17 March 2011 relation to the keeping of animals for fur production. Vulpes lagopus (L., 1758) Blårev; Domesticated From breeding. Only Arctic syn. Alopex lagopus (L., Arctic Fox, Polar Fox Fox/Polar Fox and 1758) crossings between Arctic Fox/Polar Fox and Silver Fox for fur farming when the keeping is carried out in accordance with the Regulations 17 March 2011 relation to the keeping of animals for fur production. Chinchillidae Chinchillafamilien; Chinchillas and Viscachas Chinchilla lanigera Chinchilla; Chinchilla, Domesticated. (Molina, 1782) Long-tailed Chinchilla 1 Appendix II – organisms that can be imported without a permit, see section 7 (1) a) General condition: The import shall be carried out in accordance with the due care requirements set out in Chapter V and the rest of the Regulations, as well as other applicable law, including the Regulations of 15 November 2002 No.