Appendix. Spider Phylogeny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography of the Caribbean Cyrtognatha Spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J
www.nature.com/scientificreports OPEN Biogeography of the Caribbean Cyrtognatha spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J. Binford3 & Matjaž Kuntner 1,4,5,6 Island systems provide excellent arenas to test evolutionary hypotheses pertaining to gene fow and Received: 23 July 2018 diversifcation of dispersal-limited organisms. Here we focus on an orbweaver spider genus Cyrtognatha Accepted: 1 November 2018 (Tetragnathidae) from the Caribbean, with the aims to reconstruct its evolutionary history, examine Published: xx xx xxxx its biogeographic history in the archipelago, and to estimate the timing and route of Caribbean colonization. Specifcally, we test if Cyrtognatha biogeographic history is consistent with an ancient vicariant scenario (the GAARlandia landbridge hypothesis) or overwater dispersal. We reconstructed a species level phylogeny based on one mitochondrial (COI) and one nuclear (28S) marker. We then used this topology to constrain a time-calibrated mtDNA phylogeny, for subsequent biogeographical analyses in BioGeoBEARS of over 100 originally sampled Cyrtognatha individuals, using models with and without a founder event parameter. Our results suggest a radiation of Caribbean Cyrtognatha, containing 11 to 14 species that are exclusively single island endemics. Although biogeographic reconstructions cannot refute a vicariant origin of the Caribbean clade, possibly an artifact of sparse outgroup availability, they indicate timing of colonization that is much too recent for GAARlandia to have played a role. Instead, an overwater colonization to the Caribbean in mid-Miocene better explains the data. From Hispaniola, Cyrtognatha subsequently dispersed to, and diversifed on, the other islands of the Greater, and Lesser Antilles. Within the constraints of our island system and data, a model that omits the founder event parameter from biogeographic analysis is less suitable than the equivalent model with a founder event. -

Cryptic Species Delimitation in the Southern Appalachian Antrodiaetus
Cryptic species delimitation in the southern Appalachian Antrodiaetus unicolor (Araneae: Antrodiaetidae) species complex using a 3RAD approach Lacie Newton1, James Starrett1, Brent Hendrixson2, Shahan Derkarabetian3, and Jason Bond4 1University of California Davis 2Millsaps College 3Harvard University 4UC Davis May 5, 2020 Abstract Although species delimitation can be highly contentious, the development of reliable methods to accurately ascertain species boundaries is an imperative step in cataloguing and describing Earth's quickly disappearing biodiversity. Spider species delimi- tation remains largely based on morphological characters; however, many mygalomorph spider populations are morphologically indistinguishable from each other yet have considerable molecular divergence. The focus of our study, Antrodiaetus unicolor species complex which contains two sympatric species, exhibits this pattern of relative morphological stasis with considerable genetic divergence across its distribution. A past study using two molecular markers, COI and 28S, revealed that A. unicolor is paraphyletic with respect to A. microunicolor. To better investigate species boundaries in the complex, we implement the cohesion species concept and employ multiple lines of evidence for testing genetic exchangeability and ecological interchange- ability. Our integrative approach includes extensively sampling homologous loci across the genome using a RADseq approach (3RAD), assessing population structure across their geographic range using multiple genetic clustering analyses that include STRUCTURE, PCA, and a recently developed unsupervised machine learning approach (Variational Autoencoder). We eval- uate ecological similarity by using large-scale ecological data for niche-based distribution modeling. Based on our analyses, we conclude that this complex has at least one additional species as well as confirm species delimitations based on previous less comprehensive approaches. -

Araneae, Anapidae)
Proc. 16th Europ. ColI. Arachnol. 151-164 Siedlce, 10.03.1997 Egg sac structure and further biological observations in Comaroma simonii1 Bertkau (Araneae, Anapidae) Christian KROPF Natural History Museum Berne, Department oflnvertebrates, Bernastrasse 15, CH-3005 Berne, Switzerland. Key words: Araneae, Anapidae, Comaroma, behaviour, ecology, reproduction. ABSTRACT Specimens of Comaroma simonii Bertkau from Styria (Austria) were kept in the laboratory in order to investigate biological details. Egg sacs were built at the end of June and the beginning of July. They were white-coloured, round in shape with a diameter of 1.47 mm on the average (n = 5) and were attached to vertical surfaces. Each egg sac contained three eggs of pale yellow colour. Normally the egg sac is protected by a silken funnel ending in a tube that points toward the ground underneath. It is assumed that this functions as a means of protection against egg predators and parasites. Spiderlings hatched after 27 days; they most probably moulted twice before leaving the cocoon on the 35th day. They built webs closely resembling those of the adults. Juveniles and sub adults showed no sclerotization of the body and were rarely found in the natural.habitat. There, vertical and horizontal migrations probably occur as a means of avoiding wetness or drying out, respectively. The sex ratio of all collected specimens was 98 females to 54 males. C. simonii is regarded as a 'k-strategist' and an eurychronous species. INTRODUCTION The biology of Anapidae is insufficiently known. For example, data on egg sacs or juveniles are fragmentary (Hickman 1938, 1943; Platnick & Shadab 1978; Coddington 1986; Eberhard 1987). -

Sexual Selection Research on Spiders: Progress and Biases
Biol. Rev. (2005), 80, pp. 363–385. f Cambridge Philosophical Society 363 doi:10.1017/S1464793104006700 Printed in the United Kingdom Sexual selection research on spiders: progress and biases Bernhard A. Huber* Zoological Research Institute and Museum Alexander Koenig, Adenauerallee 160, 53113 Bonn, Germany (Received 7 June 2004; revised 25 November 2004; accepted 29 November 2004) ABSTRACT The renaissance of interest in sexual selection during the last decades has fuelled an extraordinary increase of scientific papers on the subject in spiders. Research has focused both on the process of sexual selection itself, for example on the signals and various modalities involved, and on the patterns, that is the outcome of mate choice and competition depending on certain parameters. Sexual selection has most clearly been demonstrated in cases involving visual and acoustical signals but most spiders are myopic and mute, relying rather on vibrations, chemical and tactile stimuli. This review argues that research has been biased towards modalities that are relatively easily accessible to the human observer. Circumstantial and comparative evidence indicates that sexual selection working via substrate-borne vibrations and tactile as well as chemical stimuli may be common and widespread in spiders. Pattern-oriented research has focused on several phenomena for which spiders offer excellent model objects, like sexual size dimorphism, nuptial feeding, sexual cannibalism, and sperm competition. The accumulating evidence argues for a highly complex set of explanations for seemingly uniform patterns like size dimorphism and sexual cannibalism. Sexual selection appears involved as well as natural selection and mechanisms that are adaptive in other contexts only. Sperm competition has resulted in a plethora of morpho- logical and behavioural adaptations, and simplistic models like those linking reproductive morphology with behaviour and sperm priority patterns in a straightforward way are being replaced by complex models involving an array of parameters. -

T.C. Niğde Ömer Halisdemir Üniversitesi Fen Bilimleri Enstitüsü Biyoloji Anabilim Dali
T.C. Z, DEMİR, 2017 Z, DEMİR, NİĞDE ÖMER HALİSDEMİR ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ BİYOLOJİ ANABİLİM DALI ACULEPEIRA CEROPEGIA (WALCKENEAR, 1802) (ARANEAE: ARANEIDAE) TÜRÜNDE KİTİN VE KİTOSAN İZOLASYONU VE FİZİKOKİMYASAL KARAKTERİZASYONU YÜKSEK LİSANS TEZİ LİSANS YÜKSEK ZEHRA DEMİR FEN BİLİMLERİ ENSTİTÜSÜ BİLİMLERİ FEN ÖMER HALİSDEMİR ÜNİVERSİTESİ HALİSDEMİR ÖMER Eylül 2017 NİĞDE NİĞDE T.C. NİĞDE ÖMER HALİSDEMİR ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ BİYOLOJİ ANABİLİM DALI ACULEPEIRA CEROPEGIA (WALCKENEAR, 1802) (ARANEAE: ARANEIDAE) TÜRÜNDE KİTİN VE KİTOSAN İZOLASYONU VE FİZİKOKİMYASAL KARAKTERİZASYONU ZEHRA DEMİR Yüksek Lisans Tezi Danışman Doç. Dr. Osman SEYYAR Eylül 2017 1 TEZ BİLDİRİMİ Tez içindeki bütün bilgilerin bilimsel ve akademik kurallar çerçevesinde elde edilerek sunulduğunu, ayrıca tez yazım kurallarına uygun olarak hazırlanan bu çalışmada bana ait olmayan her türlü ifade ve bilginin kaynağına eksiksiz atıf yapıldığını bildiririm. Zehra DEMİR 2 ÖZET ACULEPEIRA CEROPEGIA (WALCKENEAR, 1802) (ARANEAE: ARANEIDAE) TÜRÜNDE KİTİN VE KİTOSAN İZOLASYONU VE FİZİKOKİMYASAL KARAKTERİZASYONU DEMİR, Zehra Niğde Ömer Halisdemir Üniversitesi Fen Bilimleri Enstitüsü Biyoloji Anabilim Dalı Danışman : Doç. Dr. Osman SEYYAR Eylül 2017, 32 sayfa Kitin ve kitosan son zamanlarda endüstri alanında oldukça dikkat çekmektedir ve ilaç endüstrisi, eczacılık, gıda mühendisliği, biyokatalizör, atık su temizliği gibi pek çok alanlarda kullanılmaktadır. Kitin endüstriyel olarak yengeç, karides ve istakoz gibi deniz ürünlerinden yan sanayi olarak üretilmektedir. -

A Summary List of Fossil Spiders
A summary list of fossil spiders compiled by Jason A. Dunlop (Berlin), David Penney (Manchester) & Denise Jekel (Berlin) Suggested citation: Dunlop, J. A., Penney, D. & Jekel, D. 2010. A summary list of fossil spiders. In Platnick, N. I. (ed.) The world spider catalog, version 10.5. American Museum of Natural History, online at http://research.amnh.org/entomology/spiders/catalog/index.html Last udated: 10.12.2009 INTRODUCTION Fossil spiders have not been fully cataloged since Bonnet’s Bibliographia Araneorum and are not included in the current Catalog. Since Bonnet’s time there has been considerable progress in our understanding of the spider fossil record and numerous new taxa have been described. As part of a larger project to catalog the diversity of fossil arachnids and their relatives, our aim here is to offer a summary list of the known fossil spiders in their current systematic position; as a first step towards the eventual goal of combining fossil and Recent data within a single arachnological resource. To integrate our data as smoothly as possible with standards used for living spiders, our list follows the names and sequence of families adopted in the Catalog. For this reason some of the family groupings proposed in Wunderlich’s (2004, 2008) monographs of amber and copal spiders are not reflected here, and we encourage the reader to consult these studies for details and alternative opinions. Extinct families have been inserted in the position which we hope best reflects their probable affinities. Genus and species names were compiled from established lists and cross-referenced against the primary literature. -

Araneae, Theridiidae)
Phelsuma 14; 49-89 Theridiid or cobweb spiders of the granitic Seychelles islands (Araneae, Theridiidae) MICHAEL I. SAARISTO Zoological Museum, Centre for Biodiversity University of Turku,FIN-20014 Turku FINLAND [micsaa@utu.fi ] Abstract. - This paper describes 8 new genera, namely Argyrodella (type species Argyrodes pusillus Saaristo, 1978), Bardala (type species Achearanea labarda Roberts, 1982), Nanume (type species Theridion naneum Roberts, 1983), Robertia (type species Theridion braueri (Simon, 1898), Selimus (type species Theridion placens Blackwall, 1877), Sesato (type species Sesato setosa n. sp.), Spinembolia (type species Theridion clabnum Roberts, 1978), and Stoda (type species Theridion libudum Roberts, 1978) and one new species (Sesato setosa n. sp.). The following new combinations are also presented: Phycosoma spundana (Roberts, 1978) n. comb., Argyrodella pusillus (Saaristo, 1978) n. comb., Rhomphaea recurvatus (Saaristo, 1978) n. comb., Rhomphaea barycephalus (Roberts, 1983) n. comb., Bardala labarda (Roberts, 1982) n. comb., Moneta coercervus (Roberts, 1978) n. comb., Nanume naneum (Roberts, 1983) n. comb., Parasteatoda mundula (L. Koch, 1872) n. comb., Robertia braueri (Simon, 1898). n. comb., Selimus placens (Blackwall, 1877) n. comb., Sesato setosa n. gen, n. sp., Spinembolia clabnum (Roberts, 1978) n. comb., and Stoda libudum (Roberts, 1978) n. comb.. Also the opposite sex of four species are described for the fi rst time, namely females of Phycosoma spundana (Roberts, 1978) and P. menustya (Roberts, 1983) and males of Spinembolia clabnum (Roberts, 1978) and Stoda libudum (Roberts, 1978). Finally the morphology and terminology of the male and female secondary genital organs are discussed. Key words. - copulatory organs, morphology, Seychelles, spiders, Theridiidae. INTRODUCTION Theridiids or comb-footed spiders are very variable in general apperance often with considerable sexual dimorphism. -

Tarantulas and Social Spiders
Tarantulas and Social Spiders: A Tale of Sex and Silk by Jonathan Bull BSc (Hons) MSc ICL Thesis Presented to the Institute of Biology of The University of Nottingham in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy The University of Nottingham May 2012 DEDICATION To my parents… …because they both said to dedicate it to the other… I dedicate it to both ii ACKNOWLEDGEMENTS First and foremost I would like to thank my supervisor Dr Sara Goodacre for her guidance and support. I am also hugely endebted to Dr Keith Spriggs who became my mentor in the field of RNA and without whom my understanding of the field would have been but a fraction of what it is now. Particular thanks go to Professor John Brookfield, an expert in the field of biological statistics and data retrieval. Likewise with Dr Susan Liddell for her proteomics assistance, a truly remarkable individual on par with Professor Brookfield in being able to simplify even the most complex techniques and analyses. Finally, I would really like to thank Janet Beccaloni for her time and resources at the Natural History Museum, London, permitting me access to the collections therein; ten years on and still a delight. Finally, amongst the greats, Alexander ‘Sasha’ Kondrashov… a true inspiration. I would also like to express my gratitude to those who, although may not have directly contributed, should not be forgotten due to their continued assistance and considerate nature: Dr Chris Wade (five straight hours of help was not uncommon!), Sue Buxton (direct to my bench creepy crawlies), Sheila Keeble (ventures and cleans where others dare not), Alice Young (read/checked my thesis and overcame her arachnophobia!) and all those in the Centre for Biomolecular Sciences. -

Overview of the Anyphaenids (Araneae, Anyphaeninae, Anyphaenidae) Spider Fauna from the Chocó Forest of Ecuador, with the Description of Thirteen New Species
European Journal of Taxonomy 255: 1–50 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2016.255 www.europeanjournaloftaxonomy.eu 2016 · Dupérré N. & Tapia E. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:0E8DA4DC-FF4C-436E-94FB-CB89F6416C6E Overview of the Anyphaenids (Araneae, Anyphaeninae, Anyphaenidae) spider fauna from the Chocó forest of Ecuador, with the description of thirteen new species Nadine DUPÉRRÉ 1,* & Elicio TAPIA 2 1 Research Associate, Fundación OTONGA, Calle Rither y Bolivia, Quito, Ecuador, and Research Associate, American Museum of Natural History, New York, NY, U.S.A. 2 Researcher, Centro Jambatu de Investigación y Conservación de Anfibios, Geovanny Farina 566, San Rafael, Ecuador. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:F15E1FF2-2DF5-479A-AD10-8076CE96E911 2 urn:lsid:zoobank.org:author:E842405B-5E5B-43AB-8BCD-586657AD5CFC Abstract. The spider diversity of the family Anyphaenidae in premontane, low evergreen montane and cloud forest from the Chocó region of Ecuador is examined. A total of 287 adult specimens were collected and 19 morphospecies were identified based on male specimens. Thirteen new species are described and one new genus is proposed. Five new species are described in the genus Katissa Brescovit, 1997: Katissa kurusiki sp. nov., K. puyu sp. nov., K. tamya sp. nov., K. yaya sp. nov. and K. guyasamini sp. nov. The new genus Shuyushka gen. nov. is proposed and includes three species: Shuyushka achachay gen. et sp. nov., S. moscai gen. et sp. nov. and S. -
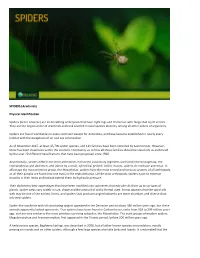
Arachnids) Physical Identification Spiders (Order Araneae
SPIDERS (Arachnids) Physical Identification Spiders (order Araneae) are air-breathing arthropods that have eight legs and chelicerae with fangs that inject venom. They are the largest order of arachnids and rank seventh in total species diversity among all other orders of organisms. Spiders are found worldwide on every continent except for Antarctica, and have become established in nearly every habitat with the exceptions of air and sea colonization. As of November 2015, at least 45,700 spider species, and 113 families have been recorded by taxonomists. However, there has been dissension within the scientific community as to how all these families should be classified, as evidenced by the over 20 different classifications that have been proposed since 1900. Anatomically, spiders differ from other arthropods in that the usual body segments are fused into two tagmata, the cephalothorax and abdomen, and joined by a small, cylindrical pedicel. Unlike insects, spiders do not have antennae. In all except the most primitive group, the Mesothelae, spiders have the most centralized nervous systems of all arthropods, as all their ganglia are fused into one mass in the cephalothorax. Unlike most arthropods, spiders have no extensor muscles in their limbs and instead extend them by hydraulic pressure. Their abdomens bear appendages that have been modified into spinnerets that extrude silk from up to six types of glands. Spider webs vary widely in size, shape and the amount of sticky thread used. It now appears that the spiral orb web may be one of the earliest forms, and spiders that produce tangled cobwebs are more abundant and diverse than orb-web spiders. -

Phylogeny of Entelegyne Spiders: Affinities of the Family Penestomidae
Molecular Phylogenetics and Evolution 55 (2010) 786–804 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny of entelegyne spiders: Affinities of the family Penestomidae (NEW RANK), generic phylogeny of Eresidae, and asymmetric rates of change in spinning organ evolution (Araneae, Araneoidea, Entelegynae) Jeremy A. Miller a,b,*, Anthea Carmichael a, Martín J. Ramírez c, Joseph C. Spagna d, Charles R. Haddad e, Milan Rˇezácˇ f, Jes Johannesen g, Jirˇí Král h, Xin-Ping Wang i, Charles E. Griswold a a Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, Golden Gate Park, San Francisco, CA 94118, USA b Department of Terrestrial Zoology, Nationaal Natuurhistorisch Museum Naturalis, Postbus 9517 2300 RA Leiden, The Netherlands c Museo Argentino de Ciencias Naturales – CONICET, Av. Angel Gallardo 470, C1405DJR Buenos Aires, Argentina d William Paterson University of New Jersey, 300 Pompton Rd., Wayne, NJ 07470, USA e Department of Zoology & Entomology, University of the Free State, P.O. Box 339, Bloemfontein 9300, South Africa f Crop Research Institute, Drnovská 507, CZ-161 06, Prague 6-Ruzyneˇ, Czech Republic g Institut für Zoologie, Abt V Ökologie, Universität Mainz, Saarstraße 21, D-55099, Mainz, Germany h Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Science, Charles University in Prague, Prague, Czech Republic i College of Life Sciences, Hebei University, Baoding 071002, China article info abstract Article history: Penestomine spiders were first described from females only and placed in the family Eresidae. Discovery Received 20 April 2009 of the male decades later brought surprises, especially in the morphology of the male pedipalp, which Revised 17 February 2010 features (among other things) a retrolateral tibial apophysis (RTA). -

Araneae) Parasite–Host Association
2006. The Journal of Arachnology 34:273–278 SHORT COMMUNICATION FIRST UNEQUIVOCAL MERMITHID–LINYPHIID (ARANEAE) PARASITE–HOST ASSOCIATION David Penney: Earth, Atmospheric and Environmental Sciences, The University of Manchester, Manchester, M13 9PL, UK. E-mail: [email protected] Susan P. Bennett: Biological Sciences, Manchester Metropolitan University, Manchester, M1 5GD, UK. ABSTRACT. The first description of a Mermithidae–Linyphiidae parasite–host association is presented. The nematode is preserved exiting the abdomen of the host, which is a juvenile Tenuiphantes species (Araneae, Linyphiidae), collected from the Isle of Mull, UK. An updated taxonomic list of known mer- mithid spider hosts is provided. The ecology of known spider hosts with regard to the direct and indirect life cycles of mermithid worms suggests that both occur in spiders. Keywords: Aranimermis, Isle of Mull, Linyphiidae, Mermithidae, Nematoda Nematode parasites of spiders are restricted to an updated and taxonomically correct list in Table the family Mermithidae but are not uncommon 1. Here we describe the first Mermithidae–Liny- (Poinar 1985, 1987) and were first reported almost phiidae parasite–host association and discuss the two and a half centuries ago (Roesel 1761). How- ecology of known spider hosts with regard to the ever, given the difficulty of identifying and rearing life cycles of mermithid worms. post-parasitic juvenile mermithids, they have re- This paper concerns three spider specimens, one ceived inadequate systematic treatment (Poinar with a worm in situ and two that are presumed to 1985). In addition, the complete life history is have been parasitized, but from which the worms known for only one species of these spider parasites have emerged and are lost.