Global Change and Human Vulnerability to Vector-Borne Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollination and Evolution of Plant and Insect Interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket a Dar, Gh
Journal of Pharmacognosy and Phytochemistry 2017; 6(3): 304-311 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Pollination and evolution of plant and insect interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket A Dar, Gh. I Hassan, Bilal A Padder, Ab R Wani and Sajad H Showket A Dar Parey Sher-e-Kashmir University of Agricultural Science and Technology, Shalimar, Jammu Abstract and Kashmir-India Flowers exploit insects to achieve pollination; at the same time insects exploit flowers for food. Insects and flowers are a partnership. Each insect group has evolved different sets of mouthparts to exploit the Gh. I Hassan food that flowers provide. From the insects' point of view collecting nectar or pollen is rather like fitting Sher-e-Kashmir University of a key into a lock; the mouthparts of each species can only exploit flowers of a certain size and shape. Agricultural Science and This is why, to support insect diversity in our gardens, we need to plant a diversity of suitable flowers. It Technology, Shalimar, Jammu is definitely not a case of 'one size fits all'. While some insects are generalists and can exploit a wide and Kashmir-India range of flowers, others are specialists and are quite particular in their needs. In flowering plants, pollen grains germinate to form pollen tubes that transport male gametes (sperm cells) to the egg cell in the Bilal A Padder embryo sac during sexual reproduction. Pollen tube biology is complex, presenting parallels with axon Sher-e-Kashmir University of guidance and moving cell systems in animals. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -

Proposed Integrated Control of Zoonotic Plasmodium Knowlesi in Southeast Asia Using Themes of One Health
Tropical Medicine and Infectious Disease Review Proposed Integrated Control of Zoonotic Plasmodium knowlesi in Southeast Asia Using Themes of One Health Jessica Scott College of Public Health and Medical and Veterinary Sciences, Australian Institute of Tropical Health and Medicine, James Cook University, Townsville 4811, Australia; [email protected] Received: 25 September 2020; Accepted: 18 November 2020; Published: 20 November 2020 Abstract: Zoonotic malaria, Plasmodium knowlesi, threatens the global progression of malaria elimination. Southeast Asian regions are fronting increased zoonotic malaria rates despite the control measures currently implemented—conventional measures to control human-malaria neglect P. knowlesi’s residual transmission between the natural macaque host and vector. Initiatives to control P. knowlesi should adopt themes of the One Health approach, which details that the management of an infectious disease agent should be scrutinized at the human-animal-ecosystem interface. This review describes factors that have conceivably permitted the emergence and increased transmission rates of P. knowlesi to humans, from the understanding of genetic exchange events between subpopulations of P. knowlesi to the downstream effects of environmental disruption and simian and vector behavioral adaptations. These factors are considered to advise an integrative control strategy that aligns with the One Health approach. It is proposed that surveillance systems address the geographical distribution and transmission clusters of P. knowlesi and enforce ecological regulations that limit forest conversion and promote ecosystem regeneration. Furthermore, combining individual protective measures, mosquito-based feeding trapping tools and biocontrol strategies in synergy with current control methods may reduce mosquito population density or transmission capacity. Keywords: Zoonotic diseases; Integrated vector management; vector-borne disease; One Health 1. -
Diversity and Evolution of Monocots
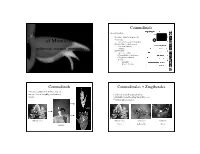
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

The Dominant Anopheles Vectors of Human Malaria in the Asia-Pacific
Sinka et al. Parasites & Vectors 2011, 4:89 http://www.parasitesandvectors.com/content/4/1/89 RESEARCH Open Access The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis Marianne E Sinka1*, Michael J Bangs2, Sylvie Manguin3, Theeraphap Chareonviriyaphap4, Anand P Patil1, William H Temperley1, Peter W Gething1, Iqbal RF Elyazar5, Caroline W Kabaria6, Ralph E Harbach7 and Simon I Hay1,6* Abstract Background: The final article in a series of three publications examining the global distribution of 41 dominant vector species (DVS) of malaria is presented here. The first publication examined the DVS from the Americas, with the second covering those species present in Africa, Europe and the Middle East. Here we discuss the 19 DVS of the Asian-Pacific region. This region experiences a high diversity of vector species, many occurring sympatrically, which, combined with the occurrence of a high number of species complexes and suspected species complexes, and behavioural plasticity of many of these major vectors, adds a level of entomological complexity not comparable elsewhere globally. To try and untangle the intricacy of the vectors of this region and to increase the effectiveness of vector control interventions, an understanding of the contemporary distribution of each species, combined with a synthesis of the current knowledge of their behaviour and ecology is needed. Results: Expert opinion (EO) range maps, created with the most up-to-date expert knowledge of each DVS distribution, were combined with a contemporary database of occurrence data and a suite of open access, environmental and climatic variables. -

Characterization of Human Body Odor and Identification of Aldehydes Using Chemical Sensor
Rev Anal Chem 2017; 36(2): 20160028 Sunil Kr. Jha* Characterization of human body odor and identification of aldehydes using chemical sensor DOI 10.1515/revac-2016-0028 to several chemical classes, including aldehyde, acid, Received April 27, 2016; accepted November 1, 2016; previously amine, alcohol, hydrocarbon, ketones, sterols, sulfur published online December 21, 2016 compounds, and terpenoids, generating human body odor (Amoore 1977, Fang et al. 1998, Toan et al. 1999, Abstract: Human body odor is a unique identity feature of Clancy and McVicar 2002, Jain 2004, Statheropoulos individual as well as an established composite of numer- et al. 2005, Havlicek and Lenochova 2006, Steeghs ous volatile organic compounds (VOCs) belonging to sig- et al. 2006, Wedekind et al. 2006, Penn et al. 2007, nificant chemical classes. Several analytical methods have D’Amico et al. 2008b, Preti and Leyden 2010, Wisthaler been used in the characterization of human body odor in and Weschler 2010, Yamazaki et al. 2010, Seeley et al. order to recognize the chemical composition of VOCs in 2011, Thorn and Greenman 2012, Agapiou et al. 2015a,b, medical, forensic, and biometric applications. Besides, Buljubasic and Buchbauer 2015, Sorokowska et al. 2015, real-time sensing systems (based on the chemical sensors) Allen et al. 2016, Colón-Crespo et al. 2016, Fialová et al. are being researched and developed for qualitative and 2016, Gildersleeve et al. 2016, Prokop-Prigge et al. 2016, quantitative recognition of VOCs in body odor. The pre- Sorokowska et al. 2016, Stefanuto and Focant 2016, sent review focuses the state-of-the-art research outcomes Verhulst et al. -

Mosquito Host Seeking in 3D Using a Versatile Climate-Controlled Wind Tunnel System
METHODS published: 11 March 2021 doi: 10.3389/fnbeh.2021.643693 Mosquito Host Seeking in 3D Using a Versatile Climate-Controlled Wind Tunnel System Annika Hinze 1, Jörgen Lantz 2, Sharon R. Hill 1,3 and Rickard Ignell 1,3* 1 Disease Vector Group, Chemical Ecology, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2 Jörgen Lantz Engineering Consulting Firm, Alnarp, Sweden, 3 Max Planck Centre Next Generation Chemical Ecology, Uppsala, Sweden Future anthropogenic climate change is predicted to impact sensory-driven behaviors. Building on recent improvements in computational power and tracking technology, we have developed a versatile climate-controlled wind tunnel system, in which to study the effect of climate parameters, including temperature, precipitation, and elevated greenhouse gas levels, on odor-mediated behaviors in insects. To establish a baseline for future studies, we here analyzed the host-seeking behavior of the major malaria vector mosquito, Anopheles gambiae sensu strico, to human odor and carbon dioxide (CO2), under tightly controlled climatic conditions, and isolated from potential background contamination by the presence of an experimenter. When presented with a combination Edited by: Bart R. H. Geurten, of human foot odor and CO2 (case study I), mosquitoes engaged in faster crosswind University of Göttingen, Germany flight, spent more time in the filamentous odor plume and targeted the odor source more Reviewed by: successfully. In contrast, female An. gambiae s. s. presented with different concentrations Merid Negash Getahun, International Centre of Insect of CO2 alone, did not display host-seeking behavior (case study II). These observations Physiology and Ecology (ICIPE), Kenya support previous findings on the role of human host-associated cues in host seeking Ivan V. -

BCIL Vector Biology PDF.Pdf
Vector Biology and Control An Update for Malaria Elimination Initiative in India Edited by Vas Dev M.Sc. (Hons.), Ph.D (Notre Dame), FNASc The National Academy of Sciences, India 2020 Vector Biology and Control: An Update for Malaria Elimination Initiative in India Edited by Vas Dev Contributors Sylvie Manguin, Vas Dev, Surya Kant Sharma, Rajpal Singh Yadav, Kamaraju Raghavendra, Poonam Sharma Velamuri, Vaishali Verma, Sreehari Uragayala, Susanta Kumar Ghosh, Khageswar Pradhan, Vijay Veer, Varun Tyagi, Manoj Kumar Das, Pradyumna Kishore Mohapatra, Ashwani Kumar, K. Hari Krishan Raju, Anupkumar Anvikar, Chazhoor John Babu, Virendra Kumar Dua, Tapan Kumar Barik, Usha Rani Acharya, Debojit Kumar Sarma, Dibya Ranjan Bhattacharyya, Anil Prakash, Nilanju Pran Sarmah Copyright © 2020 NASI Individual chapters of this book are open access under the terms of the Creative Commons Attribution 3.0 Licence (http://creativecommons.org/licenses/by/3.0) which permits users download, build upon published article, distribution, reproduction in any medium so long as the author(s) and publisher are properly credited. The author(s) have the right to reproduce their contribution in toto or part thereof for wider dissemination provided they explicitly identify the original source. All rights to the book are reserved by the National Academy of Sciences (NASI), India. The book as a whole (compilation) cannot be reproduced, distributed, or used for commercial purposes without NASI written permission. Enquiries concerning the use of the book be directed to NASI ([email protected]). Violations are liable to be prosecution under the governing Copyright law. Notice Statement and opinions expressed in the chapter are those of the contributor(s) and not necessarily those of the Editor or Publisher or the Organization. -

Sexual Systems of Plants in a Brazilian Montane Forest
Floresta e Ambiente 2019; 26(Spec No 1): e20180394 https://doi.org/10.1590/2179-8087.039418 ISSN 2179-8087 (online) Original Article Conservation of Nature Sexual Systems of Plants in a Brazilian Montane Forest Monique Perini1 , Henrique Machado Dias2 , Sustanis Horn Kunz2 1Programa de Pós-graduação em Biologia Vegetal, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Belo Horizonte/MG, Brasil 2Departamento de Ciências Florestais e da Madeira, Universidade Federal do Espírito Santo – UFES, Jerônimo Monteiro/ES, Brasil ABSTRACT In this study, vegetation reproduction has been investigated in order to understand aspects of speciation, structuring and composition of plant communities. Thus, we sought to characterize the frequency of sexual systems from species recorded in seed rain occurring in a tropical rainforest (Atlantic Forest) in Caparaó National Park, Espírito Santo State, Brazil. We collected the seed rain for twelve months, classified and recorded the species for: sexual system; pollination and dispersion syndrome; and fruit type. Then we measured the correlation between these attributes through correspondence analysis. Regarding sexual systems, 71% were hermaphrodites, 13% dioecious, and 11% monoecious. Hermaphrodites are best associated with pollination, dispersion and fruit types, represented by 65% of data variance. This study may contribute to elaborating management and conservation programs taking into account the interaction of plants with the local fauna. Keywords: seed rain, reproductive ecology, woody layer, Caparaó National Park, Atlantic Forest. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/10 Perini M, Dias HM, Kunz SH Floresta e Ambiente 2019; 26(Spec No 1): e20180394 1. -

Impacts of Deforestation on Mosquito Community Dynamics
IMPACTS OF DEFORESTATION ON MOSQUITO COMMUNITY DYNAMICS Hayley Louise Brant Imperial College London Centre for Environmental Policy, Faculty of Natural Sciences, Silwood Park Campus, Buckhurst Road, Ascot, SL5 7PY, UK Thesis submitted for the degree of Doctor of Philosophy September 2015 2 Declaration of Originality I hereby certify that all content of this thesis is my original research and collaborations with other researchers are fully acknowledged. The experimental design, data collection and analysis of Chapter 5 was completed jointly with Borame Dickens, a fellow researcher at the Centre for Environmental Policy. We both contributed equally to this chapter. Hayley Brant Names of supervisors Professor John Mumford Dr Robert Ewers Copyright declaration The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives licence. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the licence terms of this work. 3 Abstract Human-induced land use changes, including deforestation, agricultural encroachment and urbanisation, have caused widespread change in the global distribution of organisms and caused considerable declines in biodiversity through loss of habitat. Oil palm is one of the most rapidly expanding crops in Southeast Asia, but the impact of this crop on mosquito distribution, behaviour and exposure potential has been poorly explored. Understanding these factors is essential for developing, optimising and evaluating novel control measures aimed at reducing disease-transmission. -

3. the Importance of Bees in Nature
3. THE IMPORTANCE OF BEES IN NATURE BEES AS PART OF ECOSYSTEMS Pollinators strongly influence ecological relationships, ecosystem conservation and stability, genetic variation in the plant community, floral diversity, specialization and evolution. Bees play an important, but little recognized role in most terrestrial ecosystems where there is green vegetation cover for at least 3 to 4 months each year. In tropical forests, savannah woodlands, mangrove, and in temperate deciduous forests, many species of plants and animals would not survive if bees were missing. This is because the production of seeds, nuts, berries and fruits are highly dependent on insect pollination, and among the pollinating insects, bees are the major pollinators. In rain forests, especially in high mountain forests where it is too cold for most bees, other pollinators like bats and birds play a greater role in plant pollination. In farmed areas, bees are needed for the pollination of many cultivated crops (see Chapter 7), and for maintaining biodiversity in ‘islands’ of non-cultivated areas. The main role of bees in the different ecosystems is their pollination work. Other animal species are connected with bees: either because they eat the brood or honey, pollen or wax, because they are parasitic to the bees, or simply because they live within the bees nest. WHAT IS POLLINATION? Pollination is transfer of pollen from the anther (the male part of the flower) to the stigma (the female part of the flower). Some plants can pollinate themselves: in this case, the pollen passes from the anther to the stigma inside the same flower, and this is called self-pollination. -

Reproductive Ecology and Pollen Representation Among Neotropical
GEB247.fm Page 359 Tuesday, June 5, 2001 3:53 PM Global Ecology & Biogeography (2001) 10, 359–367 RESEARCH LETTER ReproductiveBlackwell Science, Ltd ecology and pollen representation among neotropical trees MARK B. BUSH1 and ROBERT RIVERA21Department of Biological Sciences, Florida Institute of Technology, 150 W. University Blvd, Melbourne, Florida 32901, U.S.A., E-mail: mbush@fit.edu 2Department of Botany, Duke University, Durham, North Carolina 27708, U.S.A. ABSTRACT species or those zoophilous species exhibiting ‘messy’ pollination syndromes. Pollination mech- Three years of pollen trapping data from Barro anisms will predictably bias the fossil record Colorado Island, Panama, are compared with local against certain flower morphologies. These data vegetation inventories. Two hypotheses relating are of significance to those using the fossil pollen pollen representation to ‘messy’ pollination and record to reconstruct the timing and sequence of flower form are tested. Dioecious taxa were found angiosperm evolution. These data help prioritize to be over-represented in pollen spectra compared plants to be included in modern pollen reference with their occurrence in local forests. Similarly, collections and to focus the search for ‘unknown’ anemophilous and ‘messy’ pollination types were types on most-likely candidate families. found to be over-represented. While anemophilous taxa were the best dispersed pollen types, zoophilous Key words anemophily, Barro Colorado Island, taxa were also well-represented in dispersal classes dioecious, hermaphroditic, monoecious, Panama, of 20–40 m and > 40 m. Thus pollen arriving to pollen representation, pollination syndrome, trop- lake sediments is most likely to be from anemophilous ical rain forest. diversity of pollen in tropical pollen rain, it is INTRODUCTION probable that an unexpectedly large proportion The description of pollination syndromes (Faegri of pollen is coming from zoophilous taxa.