Evaluation of 2-Butanone As a Substitute for Carbon Dioxide In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methyl Ethyl Ketone (2-Butanone)
Methyl Ethyl Ketone (2-Butanone) 78-93-3 Hazard Summary Methyl ethyl ketone is used as a solvent. Acute (short-term) inhalation exposure to methyl ethyl ketone in humans results in irritation to the eyes, nose, and throat. Limited information is available on the chronic (long-term) effects of methyl ethyl ketone in humans. Chronic inhalation studies in animals have reported slight neurological, liver, kidney, and respiratory effects. No information is available on the developmental, reproductive, or carcinogenic effects of methyl ethyl ketone in humans. Developmental effects, including decreased fetal weight and fetal malformations, have been reported in mice and rats exposed to methyl ethyl ketone via inhalation and ingestion. EPA has classified methyl ethyl ketone as a Group D, not classifiable as to human carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Health Effects Assessment for Methyl Ethyl Ketone (1) and EPA's Integrated Risk Information System (IRIS) (6), which contains information on inhalation chronic toxicity of methyl ethyl ketone and the RfC and oral chronic toxicity and the RfD. Uses The primary use of methyl ethyl ketone is as a solvent in processes involving gums, resins, cellulose acetate, and cellulose nitrate. (1) Methyl ethyl ketone is also used in the synthetic rubber industry, in the production of paraffin wax, and in household products such as lacquer and varnishes, paint remover, and glues. (1) Sources and Potential Exposure Methyl ethyl ketone has been detected in both indoor and outdoor air. Methyl ethyl ketone can be produced in outdoor air by the photooxidation of certain air pollutants, such as butane and other hydrocarbons. -

Methyl Isopropyl Ketone Safety Data Sheet According to Federal Register / Vol
Methyl isopropyl ketone Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 05/25/2015 Version: 1.0 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Methyl isopropyl ketone CAS-No. : 563-80-4 Product code : (US) W1814 Formula : C5H10O Synonyms : 3-Methylbutane-2-one / Isopropyl methyl ketone / Methyl-2-butanone, 3- / 2-Acetylpropane / 3- Methylbutanone-2 / 3-Methylbutanone / 3-Methylbutan-2-one / 3-Methyl-2-butanone / 2- Butanone, 3-methyl- / Butan-2-one, 3-methyl- 1.2. Recommended use and restrictions on use No additional information available 1.3. S upplie r Synerzine 5340 Hwy 42 S Ellenwood, Georgia 30294 - USA T 404-524-6744 - F 404-577-1651 [email protected] - www.synerzine.com 1.4. Emergency telephone number Emergency number : Infotrac 1-800-535-5053 (Contract# 102471) Dial +1-352-323-3500 when outside the US SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mi xt ure GHS -US classification Flammable liquids Category H225 Highly flammable liquid and vapour 2 Specific target organ toxicity H336 May cause drowsiness or dizziness (single exposure) Category 3 Hazardous to the aquatic H402 Harmful to aquatic life environment - Acute Hazard Category 3 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H225 - Highly flammable liquid and vapour H336 - May cause drowsiness or dizziness H402 - Harmful to aquatic life Precautionary statements (GHS-US) : P210 - Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. -

Toxicological Profile for 2-Butanone Released for Public Comment in May 2019
Toxicological Profile for 2-Butanone October 2020 2-BUTANONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. 2-BUTANONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -

References on Use of CO2 for Medical
Some References on the Use of CO2 for Medical Entomology Survey Becker N et al. Comparison of carbon dioxide, octenol and a host-odour as mosquito attractants in the Upper Rhine Valley, Germany. Med Vet Entomol 1995;9:377-80. Abstract: Field studies were conducted in the Upper Rhine Valley to determine the responses of mosquitoes to CDC traps baited with either CO2, octenol, light or paired combinations of these. Among eight mosquito species caught, the attractant effect on trap catches was studied in the four most abundant: Aedes vexans, Ae. rossicus, Ae. cinereus and Culex pipiens. Traps baited only with light or octenol caught few mosquitoes, whereas many were caught by traps baited with CO2 alone or in combination with either of the other candidate attractants. CO2 baited traps, with or without light, caught the most Aedes. The combination of CO2 and octenol attracted most Cx pipiens, but this apparent synergy was not significant. Using a caged hamster compared to CO2 as bait in a CDC light-trap with only intermittent fan suction, the hamster attracted less mosquitoes than CO2 emitted at a rate of 225 g/h on days 1 and 2, whereas on days 3 and 4 the smell from the hamster's cage became significantly more attractive than this rate of CO2 for all species of mosquitoes. Canyon DV, Hii JL. Efficacy of carbon dioxide, 1-octen-3-ol, and lactic acid in modified Fay- Prince traps as compared to man-landing catch of Aedes aegypti. J Am Mosq Control Assoc 1997;13:66-70. Abstract: The attractants 1-octen-3-ol and lactic acid significantly decreased catches of Aedes aegypti in Townsville, Australia, by 50% in a controlled laboratory environment and by 100% in the field when compared to carbon dioxide baited bidirectional Fay-Prince trap catches. -

Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System
insects Article Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System Dae-Yun Kim 1, Theerachart Leepasert 2, Michael J. Bangs 1 and Theeraphap Chareonviriyaphap 1,* 1 Department of Entomology, Faculty of Agriculture, Kasetsart Univeristy, Bangkok 10900, Thailand; [email protected] (D.-Y.K.); [email protected] (M.J.B.) 2 Department of Chemistry, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand; [email protected] * Correspondence: [email protected] Simple Summary: Entomological surveillance is important to evaluate vector management inter- ventions. However, collecting adult mosquitoes using direct human bait is controversial and often discouraged because of potential infection risk. Alternatively, active and passive trapping methods are available. Female mosquitoes detect human host cues such as body heat, carbon dioxide, and other volatile body emanations using olfactory sensilla to direct movement to a host. Attractive chemical lures have been identified and evaluated using a variety of olfactometric methods to in- crease trap production and efficiency. In this study, we evaluated a simple olfactometer without need of airflow. To ‘optimize’ a commercial mosquito attractant, 10 different doses of product, the TM Biogents-lure (BG-lure ), were compared. Results showed dose-dependent responses with 0.005 g with the highest attraction for Aedes aegypti, while doses of 0.2 g and above produced a repellent Citation: Kim, D.-Y.; Leepasert, T.; response. There was no significantly different response behavior between permethrin-susceptible Bangs, M.J.; Chareonviriyaphap, T. and -resistant Ae. aegypti. Culex quinquefasciatus showed significantly different responses compared Dose–Response Assay for Synthetic to Ae. -
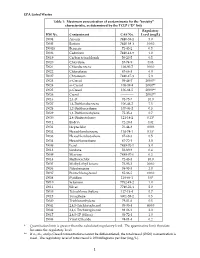
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Characterization of Human Body Odor and Identification of Aldehydes Using Chemical Sensor
Rev Anal Chem 2017; 36(2): 20160028 Sunil Kr. Jha* Characterization of human body odor and identification of aldehydes using chemical sensor DOI 10.1515/revac-2016-0028 to several chemical classes, including aldehyde, acid, Received April 27, 2016; accepted November 1, 2016; previously amine, alcohol, hydrocarbon, ketones, sterols, sulfur published online December 21, 2016 compounds, and terpenoids, generating human body odor (Amoore 1977, Fang et al. 1998, Toan et al. 1999, Abstract: Human body odor is a unique identity feature of Clancy and McVicar 2002, Jain 2004, Statheropoulos individual as well as an established composite of numer- et al. 2005, Havlicek and Lenochova 2006, Steeghs ous volatile organic compounds (VOCs) belonging to sig- et al. 2006, Wedekind et al. 2006, Penn et al. 2007, nificant chemical classes. Several analytical methods have D’Amico et al. 2008b, Preti and Leyden 2010, Wisthaler been used in the characterization of human body odor in and Weschler 2010, Yamazaki et al. 2010, Seeley et al. order to recognize the chemical composition of VOCs in 2011, Thorn and Greenman 2012, Agapiou et al. 2015a,b, medical, forensic, and biometric applications. Besides, Buljubasic and Buchbauer 2015, Sorokowska et al. 2015, real-time sensing systems (based on the chemical sensors) Allen et al. 2016, Colón-Crespo et al. 2016, Fialová et al. are being researched and developed for qualitative and 2016, Gildersleeve et al. 2016, Prokop-Prigge et al. 2016, quantitative recognition of VOCs in body odor. The pre- Sorokowska et al. 2016, Stefanuto and Focant 2016, sent review focuses the state-of-the-art research outcomes Verhulst et al. -

Methyl Ethyl Ketone
Shell Chemicals Technical Datasheet Methyl Ethyl Ketone Product Code S2113 Region Global Product Category Ketones CAS Registry Number 78-93-3 Synonym(s) 2-Butanone, MEK Description Methyl ethyl ketone, MEK, a low boiling, fast evaporating solvent is a Methyl ethyl ketone, MEK, is a low boiling, fast evaporating solvent colourless, stable liquid, partially miscible with water. MEK has exceptionally good solvency for many synthetic and natural resins that are used in the formulation of printing inks, lacquers, and other types of coatings. Typical Properties Property Unit Method Value Purity, min. %m/m GC 99.5 Water %m/m ASTM D1364 0.03 Acidity (as Acetic Acid) %m/m ASTM D1613 0.002 Density at 20°C kg/l ASTM D4052 0.805 Specific Gravity at 20°C/20°C - ASTM D4052 0.806 Specific Gravity at 25°C/25°C - ASTM D4052 0.802 Coefficient of Cubic Expansion at 20°C 10-4/°C Calculated 13 Refractive Index at 20°C - ASTM D1218 1.379 Colour Pt-Co ASTM D1209 < 5 Boiling Point °C - 80 Relative Evaporation Rate (nBuAc=1) - ASTM D3539 4.0 Relative Evaporation Rate (Ether=1) - DIN 53170 3.3 Antoine Constant A # kPa. °C - 6.18444 Antoine Constant B # kPa. °C - 1259.22 Antoine Constant C # kPa. °C - 221.758 26 Methyl Ethyl Ketone March 2016 Temperature Limits for Antoine Equation # °C - -40 to +90 Vapour Pressure at 20°C kPa Calculated 9.5 Vapour Pressure at 50°C kPa Calculated 36 Saturated Vapor Concentration at 20°C g/m3 Calculated 280 Volatile Organic Compound (VOC) g/l EU / EPA 805 Flash Point (Abel) °C IP 170 -6 Auto Ignition Temperature °C ASTM E659 515 -

SAFETY DATA SHEET Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 1
SAFETY DATA SHEET Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 1. Identification GHS product identifier : Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Other means of : Not available. identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. SDS # : 021930 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 1 substance or mixture ACUTE TOXICITY (inhalation) - Category 4 SKIN IRRITATION - Category 2 EYE IRRITATION - Category 2A TOXIC TO REPRODUCTION (Fertility) - Category 2 TOXIC TO REPRODUCTION (Unborn child) - Category 2 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Respiratory tract irritation) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) - Category 2 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Extremely flammable liquid and vapor. Harmful if inhaled. Causes serious eye irritation. Causes skin irritation. Suspected of damaging fertility or the unborn child. May cause respiratory irritation. May cause drowsiness or dizziness. May cause damage to organs through prolonged or repeated exposure. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Date of issue/Date of revision : 11/21/2017 Date of previous issue : No previous validation Version : 1 1/14 Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 2. -

Mosquito Host Seeking in 3D Using a Versatile Climate-Controlled Wind Tunnel System
METHODS published: 11 March 2021 doi: 10.3389/fnbeh.2021.643693 Mosquito Host Seeking in 3D Using a Versatile Climate-Controlled Wind Tunnel System Annika Hinze 1, Jörgen Lantz 2, Sharon R. Hill 1,3 and Rickard Ignell 1,3* 1 Disease Vector Group, Chemical Ecology, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2 Jörgen Lantz Engineering Consulting Firm, Alnarp, Sweden, 3 Max Planck Centre Next Generation Chemical Ecology, Uppsala, Sweden Future anthropogenic climate change is predicted to impact sensory-driven behaviors. Building on recent improvements in computational power and tracking technology, we have developed a versatile climate-controlled wind tunnel system, in which to study the effect of climate parameters, including temperature, precipitation, and elevated greenhouse gas levels, on odor-mediated behaviors in insects. To establish a baseline for future studies, we here analyzed the host-seeking behavior of the major malaria vector mosquito, Anopheles gambiae sensu strico, to human odor and carbon dioxide (CO2), under tightly controlled climatic conditions, and isolated from potential background contamination by the presence of an experimenter. When presented with a combination Edited by: Bart R. H. Geurten, of human foot odor and CO2 (case study I), mosquitoes engaged in faster crosswind University of Göttingen, Germany flight, spent more time in the filamentous odor plume and targeted the odor source more Reviewed by: successfully. In contrast, female An. gambiae s. s. presented with different concentrations Merid Negash Getahun, International Centre of Insect of CO2 alone, did not display host-seeking behavior (case study II). These observations Physiology and Ecology (ICIPE), Kenya support previous findings on the role of human host-associated cues in host seeking Ivan V. -
SAFETY DATA SHEET Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 1
SAFETY DATA SHEET Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 015866 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 Emergency telephone : 1-866-734-3438 number (with hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight when ambient temperature exceeds 52°C/125°F. Store in a well- ventilated place. Disposal : Not applicable. Hazards not otherwise : None known. classified Date of issue/Date of revision : 10/19/2015 Date of previous issue : No previous validation Version : 1 1/13 Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 3. -

DETERMINATION of BUTANONE CONCENTRATION in NAIL POLISH REMOVER USING a PROGRAMMABLE MBED CONTROLLER by Prince Ahmed
DETERMINATION OF BUTANONE CONCENTRATION IN NAIL POLISH REMOVER USING A PROGRAMMABLE MBED CONTROLLER by Prince Ahmed A Thesis Submitted to the Faculty of The Harriet L. Wilkes Honors College in Partial Fulfillment of the Requirements for the Degree of Bachelor of Arts in Liberal Arts and Sciences with a Concentration in Chemistry Wilkes Honors College of Florida Atlantic University Jupiter, Florida May 2018 DETERMINATION OF BUTANONE CONCENTRATION IN NAIL POLISH REMOVER USING A PROGRAMMABLE MBED CONTROLLER by Prince Ahmed This thesis was prepared under the direction of the candidate’s thesis advisor, Dr. Eugene T. Smith, and has been approved by members of his supervisory committee. It was submitted to the faculty of The Honors College and was accepted in partial fulfillment of the requirements for the degree of Bachelor of Arts in Liberal Arts and Sciences. SUPERVISORY COMMITTEE: ___________________________ Dr. Eugene T. Smith ___________________________ Dr. Shree Kundalkar ___________________________ Dean Ellen Goldey, Wilkes Honors College ___________ Date ii ACKNOWLEDGEMENTS I would like to thank Dr. Eugene T. Smith, my academic and thesis advisor for his patient advice during the course of this project. I would also like to thank Dr. Shree Kundalkar for the task of being my second reader. iii ABSTRACT Author: Prince Ahmed Title: Determination of Butanone Concentration in Nail Polish Remover Using a Programmable mBed Controller Institution: Wilkes Honors College of Florida Atlantic University Thesis Advisor: Dr. Eugene T. Smith Degree: Bachelor of Arts in Liberal Arts and Sciences Concentration: Chemistry Year: 2018 Scientific identification requires precise and quantifiable discrimination between different volatile substances in real-world samples; affordable, effective and rapid gas chromatography has been crucial towards that end (James, p.