Toxicological Profile for 2-Butanone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methyl Ethyl Ketone (2-Butanone)
Methyl Ethyl Ketone (2-Butanone) 78-93-3 Hazard Summary Methyl ethyl ketone is used as a solvent. Acute (short-term) inhalation exposure to methyl ethyl ketone in humans results in irritation to the eyes, nose, and throat. Limited information is available on the chronic (long-term) effects of methyl ethyl ketone in humans. Chronic inhalation studies in animals have reported slight neurological, liver, kidney, and respiratory effects. No information is available on the developmental, reproductive, or carcinogenic effects of methyl ethyl ketone in humans. Developmental effects, including decreased fetal weight and fetal malformations, have been reported in mice and rats exposed to methyl ethyl ketone via inhalation and ingestion. EPA has classified methyl ethyl ketone as a Group D, not classifiable as to human carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Health Effects Assessment for Methyl Ethyl Ketone (1) and EPA's Integrated Risk Information System (IRIS) (6), which contains information on inhalation chronic toxicity of methyl ethyl ketone and the RfC and oral chronic toxicity and the RfD. Uses The primary use of methyl ethyl ketone is as a solvent in processes involving gums, resins, cellulose acetate, and cellulose nitrate. (1) Methyl ethyl ketone is also used in the synthetic rubber industry, in the production of paraffin wax, and in household products such as lacquer and varnishes, paint remover, and glues. (1) Sources and Potential Exposure Methyl ethyl ketone has been detected in both indoor and outdoor air. Methyl ethyl ketone can be produced in outdoor air by the photooxidation of certain air pollutants, such as butane and other hydrocarbons. -

Methyl Isopropyl Ketone Safety Data Sheet According to Federal Register / Vol
Methyl isopropyl ketone Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 05/25/2015 Version: 1.0 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Methyl isopropyl ketone CAS-No. : 563-80-4 Product code : (US) W1814 Formula : C5H10O Synonyms : 3-Methylbutane-2-one / Isopropyl methyl ketone / Methyl-2-butanone, 3- / 2-Acetylpropane / 3- Methylbutanone-2 / 3-Methylbutanone / 3-Methylbutan-2-one / 3-Methyl-2-butanone / 2- Butanone, 3-methyl- / Butan-2-one, 3-methyl- 1.2. Recommended use and restrictions on use No additional information available 1.3. S upplie r Synerzine 5340 Hwy 42 S Ellenwood, Georgia 30294 - USA T 404-524-6744 - F 404-577-1651 [email protected] - www.synerzine.com 1.4. Emergency telephone number Emergency number : Infotrac 1-800-535-5053 (Contract# 102471) Dial +1-352-323-3500 when outside the US SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mi xt ure GHS -US classification Flammable liquids Category H225 Highly flammable liquid and vapour 2 Specific target organ toxicity H336 May cause drowsiness or dizziness (single exposure) Category 3 Hazardous to the aquatic H402 Harmful to aquatic life environment - Acute Hazard Category 3 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H225 - Highly flammable liquid and vapour H336 - May cause drowsiness or dizziness H402 - Harmful to aquatic life Precautionary statements (GHS-US) : P210 - Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. -

Toxicological Profile for 2-Butanone Released for Public Comment in May 2019
Toxicological Profile for 2-Butanone October 2020 2-BUTANONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. 2-BUTANONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -

References on Use of CO2 for Medical
Some References on the Use of CO2 for Medical Entomology Survey Becker N et al. Comparison of carbon dioxide, octenol and a host-odour as mosquito attractants in the Upper Rhine Valley, Germany. Med Vet Entomol 1995;9:377-80. Abstract: Field studies were conducted in the Upper Rhine Valley to determine the responses of mosquitoes to CDC traps baited with either CO2, octenol, light or paired combinations of these. Among eight mosquito species caught, the attractant effect on trap catches was studied in the four most abundant: Aedes vexans, Ae. rossicus, Ae. cinereus and Culex pipiens. Traps baited only with light or octenol caught few mosquitoes, whereas many were caught by traps baited with CO2 alone or in combination with either of the other candidate attractants. CO2 baited traps, with or without light, caught the most Aedes. The combination of CO2 and octenol attracted most Cx pipiens, but this apparent synergy was not significant. Using a caged hamster compared to CO2 as bait in a CDC light-trap with only intermittent fan suction, the hamster attracted less mosquitoes than CO2 emitted at a rate of 225 g/h on days 1 and 2, whereas on days 3 and 4 the smell from the hamster's cage became significantly more attractive than this rate of CO2 for all species of mosquitoes. Canyon DV, Hii JL. Efficacy of carbon dioxide, 1-octen-3-ol, and lactic acid in modified Fay- Prince traps as compared to man-landing catch of Aedes aegypti. J Am Mosq Control Assoc 1997;13:66-70. Abstract: The attractants 1-octen-3-ol and lactic acid significantly decreased catches of Aedes aegypti in Townsville, Australia, by 50% in a controlled laboratory environment and by 100% in the field when compared to carbon dioxide baited bidirectional Fay-Prince trap catches. -

Hydroxyethoxyphenyl Butanone’ (HEPB)
SCCS/1582/16 Final version Scientific Committee on Consumer Safety SCCS OPINION ON Ethylzingerone – ‘Hydroxyethoxyphenyl Butanone’ (HEPB) (Cosmetics Europe No P98) The SCCS adopted this Opinion by written procedure on 7 April 2017 SCCS/1582/16 Final Opinion on Ethylzingerone - ‘Hydroxyethoxyphenyl Butanone’ (HEPB) - Cosmetics Europe No P98 About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide Opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Ulrike Bernauer, Laurent Bodin, Leonardo Celleno, Qasim Chaudhry, Pieter Jan Coenraads, Maria Dusinska, Janine Ezendam, Eric Gaffet, Corrado Lodovico Galli, Berit Granum, Eirini Panteri, Vera Rogiers, Christophe Rousselle, Maciej Stepnik, Tamara Vanhaecke, Susan Wijnhoven. -

Critical Evaluation of Thermochemical Properties of C1–C4 Species; Updated Group-Contributions to Estimate Thermochemical Properties
Provided by the author(s) and NUI Galway in accordance with publisher policies. Please cite the published version when available. Critical evaluation of thermochemical properties of C-1-C-4 Title species: updated group-contributions to estimate thermochemical properties Author(s) Burke, S. M.; Simmie, J. M.; Curran, Henry J. Publication Date 2015-01-05 Burke, S. M., Simmie, J. M., & Curran, H. J. (2015). Critical Publication evaluation of thermochemical properties of C-1-C-4 species: Information updated group-contributions to estimate thermochemical properties. Journal of Physical and Chemical Reference Data, 44(1), 013101. Publisher AIP Publishing Link to publisher's http://dx.doi.org/10.1063/1.4902535 version Item record http://hdl.handle.net/10379/6119 DOI http://dx.doi.org/10.1063/1.4902535 Downloaded 2021-09-25T06:51:18Z Some rights reserved. For more information, please see the item record link above. C1–C4 thermochemical properties: updated group additivity groups Critical evaluation of thermochemical properties of C1–C4 species; updated group-contributions to estimate thermochemical properties. S. M. Burke, J. M. Simmie, and H. J. Currana) Combustion Chemistry Centre, National University of Ireland, Galway, Ireland (Dated: 26 September 2014) A review of literature enthalpies of formation and molar entropies for alkanes, alkenes, alcohols, hydroperox- ides, and their associated radicals has been compiled and critically evaluated. By comparing literature values the overall uncertainty in thermochemical properties of small hydrocarbons and oxygenated hydrocarbons can be highlighted. In general there is good agreement between heat of formation values in the literature for stable species, however there is greater uncertainty in the values for radical species and for molar entropy values. -

Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System
insects Article Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System Dae-Yun Kim 1, Theerachart Leepasert 2, Michael J. Bangs 1 and Theeraphap Chareonviriyaphap 1,* 1 Department of Entomology, Faculty of Agriculture, Kasetsart Univeristy, Bangkok 10900, Thailand; [email protected] (D.-Y.K.); [email protected] (M.J.B.) 2 Department of Chemistry, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand; [email protected] * Correspondence: [email protected] Simple Summary: Entomological surveillance is important to evaluate vector management inter- ventions. However, collecting adult mosquitoes using direct human bait is controversial and often discouraged because of potential infection risk. Alternatively, active and passive trapping methods are available. Female mosquitoes detect human host cues such as body heat, carbon dioxide, and other volatile body emanations using olfactory sensilla to direct movement to a host. Attractive chemical lures have been identified and evaluated using a variety of olfactometric methods to in- crease trap production and efficiency. In this study, we evaluated a simple olfactometer without need of airflow. To ‘optimize’ a commercial mosquito attractant, 10 different doses of product, the TM Biogents-lure (BG-lure ), were compared. Results showed dose-dependent responses with 0.005 g with the highest attraction for Aedes aegypti, while doses of 0.2 g and above produced a repellent Citation: Kim, D.-Y.; Leepasert, T.; response. There was no significantly different response behavior between permethrin-susceptible Bangs, M.J.; Chareonviriyaphap, T. and -resistant Ae. aegypti. Culex quinquefasciatus showed significantly different responses compared Dose–Response Assay for Synthetic to Ae. -
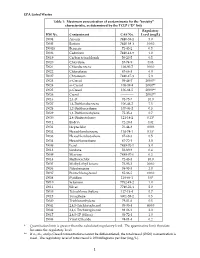
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Appendix D MEK Product Brochure
Appendix D MEK Product Brochure PRODUCT DESCRIPTION 1 of 2 Methyl Ethyl Ketone (2-Butanone, MEK) O ll MW = 72.11 CH3CCH2CH3 Methyl Ethyl Ketone, Chemical Abstracts Registry Number 78-93-3 Wiswesser Line Formula Chemical Notation 2V1 Methyl Ethyl Ketone (MEK) is a accounts for its largest use. Other important applications are as clear, colorless, low-boiling organ- Nitrocellulose lacquers consume an extraction solvent for the ic liquid with a typical ketone large volumes of Methyl Ethyl dewaxing of lube oil and as an odor. It is a fast evaporating sol- Ketone and solubilization of intermediate in the production of vent with an evaporation rate simi- acrylic coatings also contributes to antioxidants, perfumes and cata- lar to ethyl acetate. Although not the overall use of Methyl Ethyl lysts. Methyl Ethyl Ketone is also as volatile as acetone, Methyl Ketone by the surface coatings used by the hard wood pulping Ethyl Ketone is similar to acetone industry. MEK is preferred as a industry and in the production of in many respects. It is miscible lacquer solvent, because high con- smokeless powder. It is routinely with most organic solvents and is centrations of resins possessing used in printing inks, degreasing an excellent solvent for most natu- superior aliphatic and aromatic and cleaning fluids and as a com- ral and synthetic resins. diluent tolerance can be readily ponent of the solvent system used achieved as low viscosity solu- in producing magnetic tape. Methyl Ethyl Ketone is a highly tions. versatile organic compound but Methyl Ethyl Ketone as a chemi- finds its greatest utility as a sol- Methyl Ethyl Ketone is also com- cal intermediate will undergo the vent in the surface coatings indus- monly used as a solvent for rubber typical reactions associated with try. -

Methyl Ethyl Ketone
Shell Chemicals Technical Datasheet Methyl Ethyl Ketone Product Code S2113 Region Global Product Category Ketones CAS Registry Number 78-93-3 Synonym(s) 2-Butanone, MEK Description Methyl ethyl ketone, MEK, a low boiling, fast evaporating solvent is a Methyl ethyl ketone, MEK, is a low boiling, fast evaporating solvent colourless, stable liquid, partially miscible with water. MEK has exceptionally good solvency for many synthetic and natural resins that are used in the formulation of printing inks, lacquers, and other types of coatings. Typical Properties Property Unit Method Value Purity, min. %m/m GC 99.5 Water %m/m ASTM D1364 0.03 Acidity (as Acetic Acid) %m/m ASTM D1613 0.002 Density at 20°C kg/l ASTM D4052 0.805 Specific Gravity at 20°C/20°C - ASTM D4052 0.806 Specific Gravity at 25°C/25°C - ASTM D4052 0.802 Coefficient of Cubic Expansion at 20°C 10-4/°C Calculated 13 Refractive Index at 20°C - ASTM D1218 1.379 Colour Pt-Co ASTM D1209 < 5 Boiling Point °C - 80 Relative Evaporation Rate (nBuAc=1) - ASTM D3539 4.0 Relative Evaporation Rate (Ether=1) - DIN 53170 3.3 Antoine Constant A # kPa. °C - 6.18444 Antoine Constant B # kPa. °C - 1259.22 Antoine Constant C # kPa. °C - 221.758 26 Methyl Ethyl Ketone March 2016 Temperature Limits for Antoine Equation # °C - -40 to +90 Vapour Pressure at 20°C kPa Calculated 9.5 Vapour Pressure at 50°C kPa Calculated 36 Saturated Vapor Concentration at 20°C g/m3 Calculated 280 Volatile Organic Compound (VOC) g/l EU / EPA 805 Flash Point (Abel) °C IP 170 -6 Auto Ignition Temperature °C ASTM E659 515 -

SAFETY DATA SHEET Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 1
SAFETY DATA SHEET Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 1. Identification GHS product identifier : Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Other means of : Not available. identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. SDS # : 021930 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 1 substance or mixture ACUTE TOXICITY (inhalation) - Category 4 SKIN IRRITATION - Category 2 EYE IRRITATION - Category 2A TOXIC TO REPRODUCTION (Fertility) - Category 2 TOXIC TO REPRODUCTION (Unborn child) - Category 2 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Respiratory tract irritation) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) - Category 2 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Extremely flammable liquid and vapor. Harmful if inhaled. Causes serious eye irritation. Causes skin irritation. Suspected of damaging fertility or the unborn child. May cause respiratory irritation. May cause drowsiness or dizziness. May cause damage to organs through prolonged or repeated exposure. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Date of issue/Date of revision : 11/21/2017 Date of previous issue : No previous validation Version : 1 1/14 Flammable Liquid Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Toluene / Water Section 2. -
SAFETY DATA SHEET Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 1
SAFETY DATA SHEET Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 015866 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 Emergency telephone : 1-866-734-3438 number (with hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight when ambient temperature exceeds 52°C/125°F. Store in a well- ventilated place. Disposal : Not applicable. Hazards not otherwise : None known. classified Date of issue/Date of revision : 10/19/2015 Date of previous issue : No previous validation Version : 1 1/13 Nonflammable Gas Mixture: Methyl Ethyl Ketone / Methyl Isobutyl Ketone / Nitrogen / Oxygen Section 3.