Invited Review: Tannins As a Potential Alternative to Antibiotics to Prevent Coliform Diarrhea in Weaned Pigs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

'Fuyu' Persimmon Fruits
Scientia Horticulturae 172 (2014) 292–299 Contents lists available at ScienceDirect Scientia Horticulturae journal homepage: www.elsevier.com/locate/scihorti The accumulation of tannins during the development of ‘Giombo’ and ‘Fuyu’ persimmon fruits ∗ Magda Andréia Tessmer, Ricardo Alfredo Kluge, Beatriz Appezzato-da-Glória University of São Paulo/ESALQ, Department of Biological Sciences, C.P. 13418-900 Piracicaba, SP, Brazil a r t i c l e i n f o a b s t r a c t Article history: Tannins are responsible for the astringency of persimmon fruits. The present study compared the develop- Received 7 November 2013 ment of ‘Giombo’ (PVA) and ‘Fuyu’ (PCNA) persimmon fruits until maturity to determine if the difference Received in revised form 16 April 2014 in astringency between the two cultivars is related to the early accumulation of tannins in the cells or Accepted 18 April 2014 to differences in the pattern of tannin accumulation during cell differentiation, cell density or the con- centrations of total and soluble tannins. Persimmon flowers and fruits were collected from a commercial Keywords: orchard in Mogi das Cruzes during the 2010–2011 harvest season at predetermined stages until the fruit Anatomy reached commercial maturity. Structural analyses were performed by light, scanning and transmission Astringency electron microscopy, and quantitative analyses of tannin cell density, tannin cell index and total and Diospyros kaki L. soluble tannins were also performed. In both cultivars, the ovary already exhibited a small number of Tannin cells tannin cells. Throughout development, the ‘Giombo’ persimmon possessed higher tannin cell density and higher levels of total and soluble tannins compared to ‘Fuyu’. -

Notes Oak News
THE NEWSLETTER OF THE INTERNATIONAL OAK SOCIETY&, VOLUME 16, NO. 1, WINTER 2012 Greek OakOak Open Days: News September 26 - October Notes 2, 2011 From the 21st century CE to the 2nd century—BCE! The next morning early we met our large tour bus and its charming and skillful driver, Grigoris, who hails from the mountain village of Gardiki not far from here. We did a bit of leisurely botanizing before we reached Perdika, our first destination of the day. There are two reasons to visit Perdika: one is the Karavostasi beach, a curving strand with golden sand, and the archaeological site of Dymokastron, a Hellenis- tic mountain-top town reached by a steep hike. The view of the beach far below was beautiful, as it must have been when the town was still inhabited. The town was destroyed in 167 BCE by a Roman army, along with most of the other towns in the vicinity, all allied with Rome’s enemy, Macedonia. The site is under active excavation, and we were able to admire the remnants of protective walls (how in the world did they get those big stones up there?), building foundations, and cisterns, which were certainly needed in case of a prolonged siege, Some members of the IOS Greek tour relaxing under the plane tree in the which Dymocastron must have experienced more than once. village square. Vitsa, Epirus, Greece. (Photo: Gert Dessoy) The site also has many living trees, including wild pears (Py- rus spinosa Vill., also known as P. amygdaliformis Vill.) and uring this early autumn week of incomparable weather, figs (Ficus carica L.) which appear to be descendants of wild Dtwelve members of the IOS, and three others who were native trees selected by the original inhabitants, as well as guests, enjoyed a truly memorable time in northern Greece. -

Eco-Pastoral Diagnosis in the Karaburun Peninsula 15 to 22 May 2016 Conclusions and Strategic Issues for Natural Protected Areas
ECO-PASTORAL DIAGNOSIS IN THE KARABURUN PENINSULA 15 TO 22 MAY 2016 CONCLUSIONS AND STRATEGIC ISSUES FOR NATURAL PROTECTED AREAS Claire Bernard*, Alice Garnier*, Chloé Lerin**, François Lerin*, Julien Marie*** (*Ciheam Montpellier, **Benevolent intern, ***Parc National des Cévennes) Ciheam Montpellier, July 2016 BiodivBalkans Project (2012-2016): In partnership for the Ecological and Pastoral Funded by : Implemented by : Diagnosis Method with: Pastoralism & Biodiversity Management in Protected Areas Strategic proposals from an Eco-Pastoral Diagnosis in the Karaburun Peninsula, Vlorë County May 2016 Executive summary Claire Bernard, Alice Garnier, Chloé Lerin, François Lerin, Julien Marie This short report is produced within the frame of the BiodivBalkans project (2012-2016). This project is dedicated to foster rural development in mountainous regions through the construction of Signs of quality and origin (SIQO). One of its main outputs was to shed the light on the pastoral and localized livestock systems in Albania and in Balkans’ surrounding countries, as a central issue for biodiversity conservation through the maintenance of High Nature Value farming systems. They are an important component of European agriculture not only for the conservation of biodiversity, but also for cultural heritage, quality products, and rural employment. The core experience of this project was (and still is) the creation of a Protected Geographical Indication on the “Hasi goat kid meat” based on stakeholders collective action and knowledge brokering. During that learning process and to effectively enforce the relation between rural development and biodiversity conservation, we used an original Ecological and Pastoral diagnosis method, imported from an EU Life+ program (Mil’Ouv, 2013-2017). This method seeks to improve pastoral resources management in a way that is both environmentally sustainable and efficient from an economic perspective. -

Oak Open Days in Czech Republic Celebrate IOS 25Th Anniversary by Shaun Haddock
Oak News & Notes The Newsletter of the International Oak Society, Volume 21, No. 2, 2017 Twenty-six participants from ten countries plus local hosts at Plaček Quercetum © Guy Sternberg Oak Open Days in Czech Republic Celebrate IOS 25th Anniversary by Shaun Haddock wenty-six participants from ten countries arrived our first “official” visit of the event in the Park itself. T to take part in the European celebration of the From the entrance, a modest garden leads into Průho- IOS’s 25th birthday at Dušan Plaček’s Quercetum nice Castle. After passing through an arch, we found near Podĕbrady in the Czech Republic. The main ourselves on a terrace overlooking a steep-sided val- event ran from early afternoon on July 21st to the af- ley with a lake, beside which was a tree of enormous ternoon of the 23rd, but some members arrived as ear- significance for Dušan and thus for oak collecting in ly as the 19th, and by the evening of the 20th there the Czech Republic. Our mentor for the entire event, was a quorum sufficient to dine together in the event Ondřej Fous, described how this Quercus imbricaria hotel, Hotel Golfi, where we lodged. After our night’s showed Dušan that oaks have great diversity of leaf stay we departed by bus the next morning to view the shape, and that a collection of oaks would be much gardens within the grounds of Prague Castle, which more rewarding in terms of interest and variety than offer superb and enticing views over the city. The Fagus, Dušan’s original preference. -

Eficiência De Extrato Tânico E/Ou Ácido Bórico Na
EFICIÊNCIA DE EXTRATO TÂNICO COMBINADO OU NÃO COM ÁCIDO BÓRICO NA PROTEÇÃO DA MADEIRA DE Ceiba pentandra CONTRA CUPIM XILÓFAGO Leandro Calegari1, Pedro Jorge Goes Lopes2, Gregório Mateus Santana3, Diego Martins Stangerlin4, Elisabeth de Oliveira5, Darci Alberto Gatto6 1Eng. Florestal, Dr., CSTR, UFCG, Patos, PB, Brasil - [email protected] 2Acadêmico de Eng. Florestal, CSTR, UFCG, Patos, PB, Brasil - [email protected] 3Eng. Florestal, Mestrando em Ciência e Tecnologia da Madeira, UFLA, Lavras, MG, Brasil - [email protected] 4Eng. Florestal, Dr., ICAA, UFMT, Sinop, MT, Brasil - [email protected] 5Enga. Florestal, Dra., CSTR, UFCG, Patos, PB, Brasil - [email protected] 6Eng. Florestal, Dr., Centro das Engenharias, UFPel, Pelotas, RS, Brasil - [email protected] Recebido para publicação: 04/09/2012 – Aceito para publicação: 08/11/2013 Resumo Dentre os métodos que vêm sendo testados para minimizar a lixiviação de compostos de boro na madeira, destaca-se sua combinação com taninos vegetais. Aos taninos vegetais é atribuída a durabilidade natural da madeira de algumas espécies, indicando sua potencialidade como preservativo natural. Neste estudo, avaliou-se o rendimento de taninos condensados provenientes da casca de Mimosa tenuiflora em extração realizada com água destilada, comparando-o ao da extração envolvendo a inclusão de sulfito de sódio, assim como a eficiência de extratos tânicos sulfitados, combinados ou não com ácido bórico, na melhoria da resistência da madeira de Ceiba pentandra ao térmita xilófago Nasutitermes corniger, por meio de ensaio de preferência alimentar. Extrato tânico obtido com a inclusão de sulfito de sódio à água teve melhor rendimento em taninos condensados. De maneira geral, a impregnação da madeira com o extrato tânico sulfitado proporcionou o mesmo comportamento quando comparada à aplicação do ácido bórico, sendo os melhores resultados verificados quando ambos foram utilizados conjuntamente. -

Zonat E Mbrojtura Detare E Bregdetare Në Shqipëri Marine and Coastal 1 Protected Areas in Albania
Zonat e mbrojtura detare e bregdetare në Shqipëri 3 Marine and Coastal UNDP ALBANIA Protected Areas Rruga “Skënderbej”, Ndërtesa Gurten, Kati II, Tiranë in Albania www.al.undp.org UNDP Albania @UNDPAlbania ZONAT E MBROJTURA DETARE E BREGDETARE NË SHQIPËRI MARINE AND COASTAL 1 PROTECTED AREAS IN ALBANIA Tiranë, 2015 Empowered lives. Resilient nations. This publication is produced by UNDP in the framework of the project ‘Improving coverage and mangement effectiveness of marine protected ar- eas in Albania’ implemented in partnership with the Ministry of Environment © 2015 AKZM/UNDP Të gjitha të drejtat të rezervuara / All rights reserved Grupi i punës / Working group: Zamir Dedej Genti Kromidha Nihat Dragoti 2 Fotot / Photos: Genti Kromidha, Ilirjan Qirjazi, Claudia Amico Hartat / Maps: Genti Kromidha, Nihat Dragoti Shtypur në / Printed by: Tipografia DOLLONJA Përmbajtja / Content 1. Peizazhi i Mbrojtur Lumi Buna - Velipojë Buna River Velipoje Protected Landscape 2. Rezerva Natyrore e Menaxhuar Kune-Vain Tale Kune Vain Tale Managed Nature Reserve 3. Rezerva Natyrore e Menaxhuar Patok Fushëkuqe Patok Fushekuqe Managed Nature Reserve 4. Rezerva Natyrore e Menaxhuar Rrushkull Rrushkull Managed Nature Reserve 5. Parku Kombetar Divjakë - Karavasta Divjaka Karavasta National Park 6. Rezerva Natyrore e Menaxhuar Pishë Poro Pishe Poro Managed Nature Reserve 7. Peizazhi i Mbrojtur Vjosë - Nartë Vjosa Narta Protected Landscape 8. Rezerva Natyrore e Menaxhuar Karaburun Karaburun Managed Nature Reserve 3 9. Parku Kombëtar Detar Karaburun Sazan Karaburun -

Book of Abstracts
8th Regional Biophysics Conference Book of Abstracts Zreče, Slovenia 16th to 20th May 2018 Organized by: #ReBiCon2018 Editors: Hana Majaron, Petra Čotar, Monika Koren, Neža Golmajer Zima Published by: Slovenian Biophysical Society Contents 1 Maps 3 2 Programme 5 3 Abstracts 15 4 Organizing and scientific committe 165 1 Maps Poster and exhibition halls: 2 Programme Wednesday, 16. 5. 2018 11:15 Registration (11:15 - 12:45) 14:00 Registration (14:00 - 19:00) 15:45 Opening System biology, bioinformatics, omics 16:00 Plenary lecture: Mario Cindrić (HR) - Identification of microorganisms by mass spectrometry 16:45 Invited lecture: Marko Djordjević (RS) - Biophysical modeling of bacterial immune system regulation 17:15 Oral: Gergely J. Szöllősi (HU) - Tissue size regulation amplifies the effect of asymmetrical cell divisions on cancer incidence Ultrafast phenomena 17:30 Plenary lecture: Janos Hajdu (HU) - X-ray Diffraction from Single Particles and Biomolecules 18:15 Contributing lecture: Petar H. Lambrev (HU) - Ultrafast energy transfer dynamics in photosynthetic light-harvesting complexes probed by two-dimensional electronic spectroscopy 18:45 Oral: Sofia M. Kapetanaki (UK) - Understanding the interplay of heme and carbon monoxide in ion channel regulation 19:15 Get-together dinner Thursday, 17. 5. 2018 8:00 Breakfast & Registration Advanced imaging and spectroscopies 8:45 Plenary lecture: Michal Cagalinec (SK) - Cell biophysics of fluorescent probes for super-resolution optical microscopy 9:30 Invited lecture: Iztok Urbančič (SI) - Advanced STED microscopy of the membrane organisation in activating T-cells 10:00 Contributing lecture: Mario Brameshuber (AT) - Monovalent T-cell antigen receptor complexes drive T-cell antigen recognition 10:30 Oral: Josef Lazar (CZ) - 2P or not 2P: single-photon vs. -

Trees of the Bible: a Cultural History by Dr
Pub. No. 43 October 2016 Trees of the Bible: A Cultural History by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia In your backyard, within parks, hidden in forests, and along roadways, are local trees related to those mentioned in the Bible. More than 36 trees are mentioned throughout the Old and New Testa- ments. Some of these trees have relatives living here in the Southeastern United States. There is significant disagreement across time about identification of tree species mentioned in the Bible. In multiple translations from many places using different sources, some authors have reached different conclusions about what specific trees were mentioned in the Bible. The Bible is not a botanical treatise, and so modern tree identification accuracy is not relevant. Ancient Land The land of the Bible 3,000 years ago was starting to experience human development pressure, soil erosion and over-grazing which would lead to the landscapes of the modern Middle East. Natural resources present in great supply of the distant past have now dwindled to isolated remnants, included many tree species. Trees mentioned in the Bible can still be found in the wild places of the Middle East today. The Middle East area of the Bible can be generally described as historic Palestine. The area of Palestine today is made of several nations and many peoples. Historic Palestine was at the Eastern end of the Mediterranean Sea where Africa, Asia, and the Mediterranean Basin meet. This area has been cross roads for plant and plant product trade over millennium. -

Mediterranean Oaks Networks
Mediterranean Oaks Network: First meeting Mediterranean Oaks Network Report of the second meeting 2-4 May 2002–Gozo, Malta M. Bozzano and J. Turok, compilers EUFORGEN European Forest Genetic Resources Programme (EUFORGEN) Mediterranean Oaks Network Report of the second meeting 2-4 May 2002–Gozo, Malta M. Bozzano and J. Turok, compilers European Forest Genetic Resources Programme (EUFORGEN) ii EUFORGEN Mediterranean Oaks Network: Second Meeting The International Plant Genetic Resources Institute (IPGRI) is an independent international scientific organization that seeks to advance the conservation and use of plant genetic diversity for the well-being of present and future generations. It is one of 16 Future Harvest Centres supported by the Consultative Group on International Agricultural Research (CGIAR), an association of public and private members who support efforts to mobilize cutting-edge science to reduce hunger and poverty, improve human nutrition and health, and protect the environment. IPGRI has its headquarters in Maccarese, near Rome, Italy, with offices in more than 20 other countries worldwide. The Institute operates through three programmes: (1) the Plant Genetic Resources Programme, (2) the CGIAR Genetic Resources Support Programme and (3) the International Network for the Improvement of Banana and Plantain (INIBAP). The international status of IPGRI is conferred under an Establishment Agreement which, by January 2003, had been signed by the Governments of Algeria, Australia, Belgium, Benin, Bolivia, Brazil, Burkina Faso, Cameroon, Chile, China, Congo, Costa Rica, Côte d’Ivoire, Cyprus, Czech Republic, Denmark, Ecuador, Egypt, Greece, Guinea, Hungary, India, Indonesia, Iran, Israel, Italy, Jordan, Kenya, Malaysia, Mauritania, Morocco, Norway, Pakistan, Panama, Peru, Poland, Portugal, Romania, Russia, Senegal, Slovakia, Sudan, Switzerland, Syria, Tunisia, Turkey, Uganda and Ukraine. -

Biorestoration Management Plan Appendix 10 – Description of Trees
Biorestoration Management Plan Appendix 10 – Description of Trees for Biorestoration TAP AG CAL00-PMT-601-Y-TTM-0002 Rev. No.: 3 Doc. no.: Biorestoration Management Plan Doc. Title: Appendix 10 – Description of Trees for Page: 1 of 7 Biorestoration Appendix 10 – Description of Trees for Biorestoration Contents 1. Description of tree species used for Reforestation ....................................................................... 2 1.2 Black alder (Alnus glutlnosa) ........................................................................................................ 2 1.3 Austrian oak (Quercus cerris) ....................................................................................................... 2 1.4 Black Poplar (Populus nigra) ........................................................................................................ 2 1.5 Bladder-senna (Colutea arborescens) .......................................................................................... 2 1.6 Bladder-senna (Colutea arborescens) .......................................................................................... 2 1.7 Corneilian cherry (Comus mas) .................................................................................................... 2 1.8 Hawthorn (Crataegus monogyna) ................................................................................................. 3 1.9 Italina cypress (Cupressus sempervirens).................................................................................... 3 1.10 Downy oak (Quercus pubescens) -
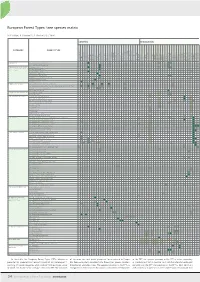
34 European Forest Types: Tree Species Matrix
M. Pividori, F. Giannetti,A.Barbati,G.Chirici M. Pividori,F. European ForestTypes:treespeciesmatrix of whicharedivided intosub-types.Forevery EFTthepresence types,some counting 14broadcategorieswhich include78forest presented as proposed and revised by Barbati andcolleagues presented asproposedandrevised by 34 coniferous forest forest coniferous andmixedbroadleaved- coniferous andnemoral 2. Hemiborealforest 1. Borealforest 14. Introducedtreespeciesforest aspen forest birch,or 13. Nonriverinealder, 12. Floodplainforest 11. Mireandswampforest Macaronesian regions Mediterranean, Anatolianand ofthe forests 10. Coniferous 9. Broadleavedevergreenforest 8. Thermophilousdeciduousforest 7. Mountainousbeechforest 6. Beechforest 5. Mesophyticdeciduousforest forest 4. Acidophilousoakandoak-birch forest 3. Alpineconiferous In this table the European Forest Types (EFTs) scheme is In thistabletheEuropeanForest Types(EFTs) CATEGORY 10.11 Mediterraneanyewstands articulatastands 10.10 Tetraclinis 10.9 Cedarforest 10.8 Cypressforest 10.7 Juniperforest 10.6 MediterraneanandAnatolianfirforest 10.5 Alti-Mediterraneanpineforest 10.4 MediterraneanandAnatolianScotspineforest 10.3 Canarianpineforest 10.2.2 AnatolianBlackpineforest 10.2.1 MediterraneanBlackpineforest -Pinuspinea 10.1.3 Mediterraneanpineforest -Pinushalepensis 10.1.2 Mediterraneanpineforest -Pinuspinaster 10.1.1 Mediterraneanpineforest 9.5 Othersclerophlyllousforests 9.4 Macaronesianlaurisilva groves 9.3 Palm 9.2 Olive-carobforest 9.1 Mediterraneanevergreenoakforest 8.8.8 Horsechestnutandwalnutmixedwoods -

Biologia Craig Heller Sallyh
David Sadava, David M. Hillis, H. Craig Heller, Sally Hacker 1 David Sadava David M. Hillis Biologia Hacker H. Heller Sally Craig M. Hillis Sadava David David 1. La cellula H. Craig Heller Sally Hacker Quinta edizione italiana condotta sulla undicesima edizione americana La biologia è in continua evoluzione: nuove ipotesi si tra- ricerche recenti; la risposta dettagliata alla domanda ducono in nuove conoscenze, ma anche in nuovi spunti si trova a fine capitolo. Biologia di ricerca e nuovi strumenti di insegnamento, e questo • L’esperimento: la descrizione della ricerca che sta alla rende l’esigenza di restare aggiornati più urgente rispet- base di Un caso da vicino. 1. La cellula to ad altre discipline. • Lavorare con i dati: una proposta di lavoro sui dati La quinta edizione italiana di Biologia raccoglie il reali dell’esperimento, nella quale lo studente è invi- patrimonio di informazioni, strumenti e prospettive tato ad analizzare i risultati da sé e a rispondere ad Quinta edizione italiana accumulato negli ultimi anni e lo organizza partendo alcune domande. condotta sulla undicesima edizione americana dall’idea che la biologia sia prima di tutto un sistema: • Prospettive future: nuove domande e opportunità di quale che sia il livello di organizzazione che si vuole ricerca, sempre in rapporto a Un caso da vicino, a fine Biologia indagare, dalle molecole agli ecosistemi, i sistemi biolo- capitolo. gici sono interconnessi e complessi, e serve un approccio • Concetti chiave: sintesi di idee portanti all’inizio di integrato. Questa constatazione legata allo studio della ogni paragrafo. disciplina riflette il fatto che la popolazione umana è con- • Ricapitoliamo: riassunto del paragrafo, con un elenco nessa in modo imprescindibile con le altre forme viventi.