6 Biochemical Reactions 67
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Biological Fluid Power Systems and Their Potential Bionic Applications
The University of Manchester Research A review of biological fluid power systems and their potential bionic applications DOI: 10.1007/s42235-019-0031-6 Document Version Accepted author manuscript Link to publication record in Manchester Research Explorer Citation for published version (APA): Liu, C., Wang, Y., Ren, L., & Ren, L. (2019). A review of biological fluid power systems and their potential bionic applications. Journal of Bionic Engineering. https://doi.org/10.1007/s42235-019-0031-6 Published in: Journal of Bionic Engineering Citing this paper Please note that where the full-text provided on Manchester Research Explorer is the Author Accepted Manuscript or Proof version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version. General rights Copyright and moral rights for the publications made accessible in the Research Explorer are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Takedown policy If you believe that this document breaches copyright please refer to the University of Manchester’s Takedown Procedures [http://man.ac.uk/04Y6Bo] or contact [email protected] providing relevant details, so we can investigate your claim. Download date:04. Oct. 2021 A review of biological fluid power systems and their potential bionic applications Chunbao Liu1,2, Yingjie Wang 1,2, Luquan Ren 2, Lei Ren2,3 1 School of Mechanical and Aerospace Engineering, Jilin University, Changchun 130022, China 2 Key Laboratory of Bionic Engineering, Ministry of Education, Jilin University, Changchun 130022, China 3 School of Mechanical, Aerospace and Civil Engineering, University of Manchester, Manchester M13 9PL, UK Abstract Nature has always inspired human achievements in industry, and biomimetics is increasingly being applied in fluid power technology. -

Chapter 1 and 2
CHAPTER 1 AND 2 Life is a unique, complex organization of molecules that expresses it self though chemical reactions which lead to growth, development, responsiveness, adaptation & reproduction. Unique features of living organesim: Growth- reproduction- metabolism- consciousness-life span. Living organisims are therefore, self- replicating, evolving & self-regulatory interactive systems capable of responding to external stimuli. Currently 1.7-1.8 billion living organisms known to science. Out of which 1.25 are animals and abut 0.5 millions are plants. Systematic is branch of biology that deals with cataloguing plants, animals and other organism into categories that can be named, compared & studied. Biology : father of biology- Aristotle,Biology (Bio-life form, logy-study) -Father of Zoology ( Aristotle ) Father of Botany ( Theophrastus ) Taxonomy: study of rules & procedure to classify organisms. Cell contains - Cytoplasm and Nucleoplasm Collectively called Protoplasm (Physical bas is of life ) given by purkinje Taxonomic categories (7 obligate ) - Kingdom (less similarities) - Phylum ( animal ) / Division (plant ) - Class - Order - Family - Genus - Species ( More similarity ) basic unit of classification Bionomical nomenclature given by C.linneaus Taxonomic AIDS: Herbarium : It is a place where dried and pressed specimens, mounted on sheets are kept systematically according bentham to bantams & hooker classification. It carries a label on right corner which provide. Information for future use. It provides a quick refer back system and is quite useful for people involved in taxonomic studies. All institutes leading with baotanical studies maintain their herbaria. HISTORY OF HERBARIA : Majority of the world’s famous herbaria originated from the botanical gardens. The first herbarium was set up at Pisa in Italy by a professor of botany Luca Ghini. -

P> TEST YOUR KNOWLEDGE Chapter 32: Introduction to Animal Evolution
TEST YOUR KNOWLEDGE Chapter 32: Introduction to Animal Evolution MULTIPLE CHOICE: Choose the one best answer. 1. Sponges differ from the rest of the animals be cause a. they are completely sessile. b. they have radial symmetry and are suspension- feeders. c -theirsimpIe-body structure rLas-notrue-nssues—-- and they have a unique embryology. d. they are not multicellular. e. they have no flagellated cells. 7. Which of the following is not descriptive of a 2.. An. insect larva pseudocoelomate? a. is a miniature version of the adult. a. a body cavity incompletely lined by mesoderm b. is transformed into an adult by molting. b. bilateral symmetry c. ensures more genetic variation in the insect life c. triploblastic cycle. d. true tissues d. is a sexually immature organism specialized e. schizocoelous formation of body cavity for eating and growth. e. is all of the above. 8. According to evidence from developmental genetics, the last common ancestor of the bilateral 3. Cephalization animals may have been a. is the development of bilateral symmetry. a. a parazoan that resembled a sponge. b. is the formation of a coelom by budding from b. a complex animal with a head, a longitudinal the archenteron. nerve cord, and a segmented body with legs. c. is a diagnostic characteristic of deuterostomes. c. a simple bilateral animal with no specializa d. is common in radially symmetrical animals. tion. e. is associated with motile animals that concen d. a colonial flagellated protist. trate sensory organs in a head region. e. a pseudocoelomate such as a roundworm. 4. A true coelom a. -

FISH310: Biology of Shellfishes
FISH310: Biology of Shellfishes Lecture Slides #3 Phylogeny and Taxonomy sorting organisms How do we classify animals? Taxonomy: naming Systematics: working out relationships among organisms Classification • All classification schemes are, in part, artificial to impose order (need to start some where using some information) – Cell number: • Acellular, One cell (_________), or More than one cell (metazoa) – Metazoa: multicellular, usu 2N, develop from blastula – Body Symmetry – Developmental Pattern (Embryology) – Evolutionary Relationship Animal Kingdom Eumetazoa: true animals Corals Anemones Parazoa: no tissues Body Symmetry • Radial symmetry • Phyla Cnidaria and Ctenophora • Known as Radiata • Any cut through center ! 2 ~ “mirror” pieces • Bilateral symmetry • Other phyla • Bilateria • Cut longitudinally to achieve mirror halves • Dorsal and ventral sides • Anterior and posterior ends • Cephalization and central nervous system • Left and right sides • Asymmetry uncommon (Porifera) Form and Life Style • The symmetry of an animal generally fits its lifestyle • Sessile or planktonic organisms often have radial symmetry • Highest survival when meet the environment equally well from all sides • Actively moving animals have bilateral symmetry • Head end is usually first to encounter food, danger, and other stimuli Developmental Pattern • Metazoa divided into two groups based on number of germ layers formed during embryogenesis – differs between radiata and bilateria • Diploblastic • Triploblastic Developmental Pattern.. • Radiata are diploblastic: two germ layers • Ectoderm, becomes the outer covering and, in some phyla, the central nervous system • Endoderm lines the developing digestive tube, or archenteron, becomes the lining of the digestive tract and organs derived from it, such as the liver and lungs of vertebrates Diploblastic http://faculty.mccfl.edu/rizkf/OCE1001/Images/cnidaria1.jpg Developmental Pattern…. -
Animal Diversity Notes
Animal Diversity Notes Key Biology Concepts Protostomes/Deuterostomes Difference based on: type of embryo cleavage fate of blastopore ontogeny of coelom (schizocely vs enterocely) Tradeoffs of Ectothermy Disadvantages: o Activity limited by external temperature o Requires behavioural adaptations for finding thermal microenvironments (basking in sun or finding cool refuge) o Become inactive for part of the year o Limitations on latitudinal range (not as far north or south as endotherms) 1 Advantages: o High efficiency of converting ingested food to biomass o Can thrive in ecosystems of low productivity (deserts, hot and dry) o Can survive with low metabolic rate Purposes of Migration broadens the resource base, tracks available food high latitudes have longer days in summer, allowing extended foraging maintains relatively constant temperature by going tropics as winter sets in prevents permanent predation pressure in one location Advantages of the Coelomate Body Plan Cavity can function for circulation, waste disposal, and gamete storage and release Enables the development of a hydrostatic skeleton Provides cushioning for the digestive tract Muscular movements of the digestive tract isolated from the outer body wall and skeletal muscular movements Advantages of Metamerism Improved efficiency of motion using hydrostatic skeleton Independent nervous control and movement of segments Architectural redundancy allows specialisation of segments as well as survival/regeneration when segments are lost Modes of Development 2 3 Timeline of Evolution 4 Essential Diagrams Cladograms 5 6 7 8 9 10 Key Animal Diagrams 11 12 13 14 15 16 17 18 19 20 21 22 23 Animal Phyla Porifera Calcarea: calcareous sponges, mostly small Hexactinellida: possess six-rayed siliceous spicules, have continuous syncytial tissue called trabecular reticulum Demospongiae: Over 80% of sponge species, all are leuconoid, can have siliceous spicules or spongin fibres Cnidaria Hydrozoa: generally have asexual polyp and sexual medusa stages (e.g. -

Glossary.Pdf
Glossary Pronunciation Key accessory fruit A fruit, or assemblage of fruits, adaptation Inherited characteristic of an organ- Pronounce in which the fleshy parts are derived largely or ism that enhances its survival and reproduc- a- as in ace entirely from tissues other than the ovary. tion in a specific environment. – Glossary Ј Ј a/ah ash acclimatization (uh-klı¯ -muh-tı¯-za -shun) adaptive immunity A vertebrate-specific Physiological adjustment to a change in an defense that is mediated by B lymphocytes ch chose environmental factor. (B cells) and T lymphocytes (T cells). It e¯ meet acetyl CoA Acetyl coenzyme A; the entry com- exhibits specificity, memory, and self-nonself e/eh bet pound for the citric acid cycle in cellular respi- recognition. Also called acquired immunity. g game ration, formed from a fragment of pyruvate adaptive radiation Period of evolutionary change ı¯ ice attached to a coenzyme. in which groups of organisms form many new i hit acetylcholine (asЈ-uh-til-ko–Ј-le¯n) One of the species whose adaptations allow them to fill dif- ks box most common neurotransmitters; functions by ferent ecological roles in their communities. kw quick binding to receptors and altering the perme- addition rule A rule of probability stating that ng song ability of the postsynaptic membrane to specific the probability of any one of two or more mu- o- robe ions, either depolarizing or hyperpolarizing the tually exclusive events occurring can be deter- membrane. mined by adding their individual probabilities. o ox acid A substance that increases the hydrogen ion adenosine triphosphate See ATP (adenosine oy boy concentration of a solution. -
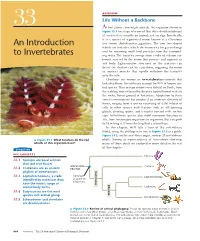
An Introduction to Invertebrates 667 � Figure 33.3 (Continued) Exploring Invertebrate Diversity
OVERVIEW Life Without a Backbone 33 At first glance, you might mistake the organism shown in Figure 33.1 for a type of seaweed. But this colorful inhabitant of coral reefs is actually an animal, not an alga. Specifically, it is a species of segmented worm known as a Christmas An Introduction tree worm (Spirobranchus giganteus). The two tree-shaped whorls are tentacles, which the worm uses for gas exchange and for removing small food particles from the surround- to Invertebrates ing water. The tentacles emerge from a tube of calcium car- bonate secreted by the worm that protects and supports its soft body. Light-sensitive structures on the tentacles can detect the shadow cast by a predator, triggering the worm to contract muscles that rapidly withdraw the tentacles into the tube. Christmas tree worms are invertebrates—animals that lack a backbone. Invertebrates account for 95% of known ani- mal species. They occupy almost every habitat on Earth, from the scalding water released by deep-sea hydrothermal vents to the rocky, frozen ground of Antarctica. Adaptation to these varied environments has produced an immense diversity of forms, ranging from a species consisting of a flat bilayer of cells to other species with features such as silk-spinning glands, pivoting spines, and tentacles covered with suction cups. Invertebrate species also show enormous variation in size, from microscopic organisms to organisms that can grow to 18 m long (1.5 times the length of a school bus). In this chapter, we’ll take a tour of the invertebrate world, using the phylogenetic tree in Figure 33.2 as a guide. -

Muscular and Hydrostatic Action in the Sea-Anemone Metridium Senile (L.)
[264] MUSCULAR AND HYDROSTATIC ACTION IN THE SEA-ANEMONE METRIDIUM SENILE (L.) BY E. J. BATHAM AND C. F. A. PANTIN Zoological Laboratory, University of Cambridge (Received i March 1950) (With Plate 7 and Nine Text-figures) The majority of sea-anemones present to the eye an almost constant shape which only changes as the result of activities of a very simple kind. This is true, for instance, of Calliactis parasitica, the subject of an earlier study (Pantin, 1935). The unstimu- lated animal rarely departs from the form of a short cylinder surmounted by a disk bearing the tentacles round its edge and the mouth at its centre. In contrast with Calliactis, Metridium senile is notable for the variety of shapes which it assumes (PL 7). It may be fully expanded with a narrow or with a wide column (e, /). The same anemone may next day be found contracted down to a mere button-like object (d). It may indulge in a variety of contortions in which it is laterally bent, or possesses deep transverse constrictions locally or completely embracing the column (g, i). The whole anemone may on occasion temporarily assume a shrivelled and contracted condition (h), in which it might easily be discarded from the aquarium as dying; but which, in fact, is a normal temporary state from which there is com- paratively rapid recovery (i, j). At other times, particularly during locomotion, a part of the body may become greatly distended (c). Prolonged observation of Calliactis shows that even here there is some variation of shape, though less evident than with Metridium. -

Phylum Cnidaria Anemones, Corals, Jellyfishes, Hydras and Relatives by John R
Lecture 2 Phylum Cnidaria Anemones, corals, jellyfishes, hydras and relatives by John R. Finnerty Animal Phylogeny Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata Nematoda Platyhelminthes Acoelomorpha Silicispongiae Calcispongia PROTOSTOMIA Phylum Cnidaria Cnidaria (Greek: “stinging thread”) distinguished by the possession of cnidae 10,000 described species—sea anemones, corals, jellyfishes and hydras diploblast = 2 germ layers (ectoderm & endoderm) blind gut (single opening) nerve net & muscle cells radial symmetry??? sexual & asexual reproduction Cnidocytes & Cnidae Diagnostic of cnidarians. However, both ctenophores (Haeckelia [=Euchlora]) and aeolid nudibranchs may re-deploy cnidae that they have obtained from their cnidarian prey. (Barnes, Invertebrate Zoology, 1987) Cnidocytes & Cnidae The cnidocyte is a sensory-effector cell containing a cnida. Each cnida is a rounded proteinaceous capsule, with an opening on the apical surface that is often covered by a hinged operculum. At the surface, where the cnida opens, there are generally found a number of modified cilia, called cnidocil, which assist in the perception of tactile stimulation and chemical stimulation. In this sac, there is a long hollow thread. Upon mechanical contact and receipt of appropriate chemical stimuli, this thread is explosively everted from the sac. The cnidae may serve to deliver venom, like a hypodermic needle. Many “nematocysts” function like this. The cnidae may -

Lab 6 – Phylum Arthropoda
Biology 18 Spring, 2008 Lab 6 – Phylum Arthropoda Objectives: Understand the taxonomic relationships and major features of arthropods Learn the external and internal anatomy of the crayfish and an insect Use the live and preserved invertebrate specimens to understand the major advantages and limitations of exoskeletons in relation to the hydrostatic skeletons of worms and the endoskeletons of vertebrates, which you will examine next week Textbook Reading: pp. 705-714, 1016, 1078 (Fig. 50.8), 978 (Fig. 45.16), 1028-1029 (Figs. 48.3 & 48.4), 1046 (Fig. 49.1), 1098 (Fig. 51.6) Introduction Arthropods are the largest group of animals, comprising over 1,000,000 terrestrial and aquatic species. By comparison, chordates (including vertebrates) number roughly 40,000 species. Arthropods were not only the first really successful group of animals to invade land, they are the most successful group of terrestrial animals. The invasion of land by arthropods 430 million years ago opened up many new niches and led to an adaptive radiation unparalleled by any group of organisms. Arthropods are found flying thousands of feet above the surface of the earth and thousands of feet below the ocean surface, and just about everywhere in between. Given their numbers and diversity, arthropods have had profound effects on the plants and animals around them, including humans. Many, especially crustaceans, are important food sources. Some insect species (butterflies, moths, bees, and flies) are necessary for the propagation of fruit and vegetable plants, and thus it is not surprising that pollinating insects evolved simultaneously with flowering plants. Others are important for pharmaceutical research: the venom of certain spiders, for example, may be useful in the treatment of Alzheimer's disease. -

Advanced Placement Biology
Advanced Placement Biology Summer Assignment Textbook: Biology 5th & 8th edition and by Campbell, Reece and Mitchell. The 8th edition book must be checked out from the library by June 8th. Assignment: Read chapters 50-53. Define the Key Terms and answer the Objective Questions for each chapter. All 3 chapters (6 assignments) are due the 1st day of school. There will be a quiz on the 2st day of school covering chapters 50-53. Join Google Classroom with the code “mexeu8” and access the pdf. Eventually the Key Terms and Objectives can be found at the Haiku website. Click on “AP Biology” and then click on “Ch. 33-55 lecture notes”. Scroll down to Ch.50. The homework will be found on the 1st or 2nd page of each chapter. Please DO NOT type any homework assignments due to rampant plagiarisms in previous years and place each of the 6 assignments on separate pages. Any questions about the assignments can be asked through my email: [email protected]. Please note that online homework chapter numbers do not align with new textbook chapter numbers due to the author dying before publishing new homework. Please realize that Advance Placement Biology is a University level biology class for biology majors. It requires a very significant commitment of time, energy, maturity and dedication to the class to succeed. I hope you have fun and safe summer, see you in September. Mr. Reta CHAPTER 33 INVERTEBRATES OUTLINE I. The Parazoa A. Phylum Porifera: sponges are sessile with porous bodies and choanocytes II. The Radiata A. -

An Evolutionary Comparative Analysis of the Medusozoan (Cnidaria) Exoskeleton
Zoological Journal of the Linnean Society, 2016, 178, 206–225. With 4 figures Downloaded from https://academic.oup.com/zoolinnean/article-abstract/178/2/206/2674323 by Universidade Estadual Paulista J�lio de Mesquita Filho user on 18 April 2019 An evolutionary comparative analysis of the medusozoan (Cnidaria) exoskeleton MARIA A. MENDOZA-BECERRIL1*, MAXIMILIANO M. MARONNA1,MIRIAN L. A. F. PACHECO2, MARCELLO G. SIMOES~ 3, JULIANA M. LEME4, LUCILIA S. MIRANDA1, ANDRE C. MORANDINI1 and ANTONIO C. MARQUES1,5 1Department of Zoology, Institute of Biosciences, University of Sao~ Paulo, Rua do Matao,~ Trav. 14, 101, 05508-090 Sao~ Paulo, Brazil 2Department of Biology, Federal University of Sao Carlos, Rodovia Joao~ Leme dos Santos - atekm 104.000 Parque Reserva Fazenda Imperial, 18052780 Sorocaba, Sao~ Paulo, Brazil 3Department of Zoology, Laboratory of Paleozoology, Sao~ Paulo State University Botucatu, Jardim Santo Inacio (Rubiao~ Junior), 18618970 Botucatu, Sao~ Paulo, Brazil 4Department of Sedimentary and Environmental Geology, Institute of Geosciences, University of Sao~ Paulo, Rua do Lago, 562, 05508-080 Sao~ Paulo, Brazil 5Center for Marine Biology, University of Sao~ Paulo, Rodovia Manoel H. Do Rego km 131.5, CEP 11600-000 Sao~ Sebastiao,~ Sao~ Paulo, Brazil Received 16 June 2015; revised 3 January 2016; accepted for publication 7 February 2016 The benthic polyp phase of Medusozoa (Staurozoa, Cubozoa, Scyphozoa, and Hydrozoa) has endoskeletal or exoskeletal support systems, but their composition, development, and evolution is poorly known. In this contribution the variation in synthesis, structure, and function of the medusozoan exoskeleton was examined. In addition, an evolutionary hypothesis for its origin and diversification is proposed for both extinct and extant medusozoans.