Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Foot–Pound–Second System
Foot–pound–second system The foot–pound–second system or FPS system is a system of units built on three fundamental units: the foot for length, the (avoirdupois) pound for either mass or force (see below), and the second for time.[1] Contents Variants Pound as mass unit Pound-force as force unit Pound as force unit Other units Molar units Electromagnetic units Units of light Conversions See also References Variants Collectively, the variants of the FPS system were the most common system in technical publications in English until the middle of the 20th century.[1] Errors can be avoided and translation between the systems facilitated by labelling all physical quantities consistently with their units. Especially in the context of the FPS system this is sometimes known as the Stroud system after William Stroud, who popularized it.[2] Three approaches to English units of mass and force or weight[3][4] Base Force Weight Mass 2nd law of m = F F = W⋅a motion a g F = m⋅a System British Gravitational (BG) English Engineering (EE) Absolute English (AE) Acceleration ft/s2 ft/s2 ft/s2 (a) Mass (m) slug pound-mass pound Force (F), pound pound-force poundal weight (W) Pressure (p) pound per square inch pound-force per square inch poundal per square foot Pound as mass unit When the pound is used as a unit of mass, the core of the coherent system is similar and functionally equivalent to the corresponding subsets of the International System of Units (SI), using metre, kilogram and second (MKS), and the earlier centimetre–gram–second system of units (CGS). -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Conversion Factors
CONVERSION FACTORS LENGTH MASS inches x 25.4 =millimetres (mm) ounce x 28.35 = grams (g) feet x 0.3048 = metres (m) pound x 0.4536 = kilograms (kg) yards x 0.9144 = metres (m) slug x 14.59 = kg chain x 20.12 = metres (m) ton (2240 lb) x 1016.05 = kg mile x 1609 = metres (m) short ton (2000 lb) x 907.2 = kg ton (2240 lb) x 1.016 = tonne (t) AREA pounds per foot Square inches x 645.16 = mm 2 (lb/ft) x 1.488 = kg/m Square feet x 0.092 = m2 pounds per yard Square yards x 0.836 = m2 (lb/yd) x 0.4961 = kg/m acre x 4047 = m2 hectare (ha) x 10,000 = m2 DENSITY lb/in x 27.68 = t/m3 VOLUME lb/ft x 16.02 = kg/m 3 cubic inches x 16,387 = mm 3 lb/yd x 0.5933 = kg/m 3 cubic feet x 0.0283 = m3 cubic yards x 0.9143 = m3 VOLUME CAPACITY cubic inch (in ) x 16387 = mm 3 VELOCITY cubic foot (ft ) x 0.02832 = m3 ft. per second (ft/s) x 0.3048 = m/s cubic yard (yd ) x 0.7646 = m3 ft. per minute(ft/min) x 0.00508 = m/s litre (L) x 1,000,000 = mm 3 miles per hour x 0.4470 = m/s litre (L) x 0.001 = m3 miles per hour x 1.609 = km/h gallons (Imp) x 0.004546 = m3 fluid ounce x 28.41 = millilitre (ml) FORCE pint (20 fl. oz) x 568.3 = millilitre (ml) Poundal (pdl) x 0.1383 = N quart (2 pints) x 1.137 = litre (L) Pound-force (lbf) x 4.448 = N gallon (Imp) x 4.546 = litre (L) ton-force (tonf) x 9.964 = kN litre (water 4°C) x 1.000 = litre (L) kilogram-force (kgf) x 9.807 = N Imp. -
Unit Systems
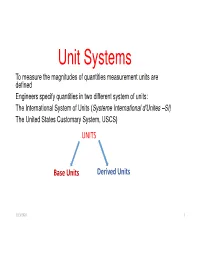
Unit Systems To measure the magnitudes of quantities measurement units are defined Engineers specify quantities in two different system of units: The International System of Units ( Systeme International d’Unites –SI) The United States Customary System, USCS) UNITS Base Units Derived Units 3/13/2020 1 BASE UNITS • A base unit is the unit of a fundamental quantity. Base units are independent of one another and they form the core of unit system. They are defined by detailed international agreements. Base Quantity (common symbol) SI Base Unit Abbreviation Length (l, x, d, h etc.) Meter m Mass (m) Kilogram kg Time (t) Second s Temperature (T) Kelvin K Electric Current (i ) Ampere A Amount of substance (n) Mole mol Luminous intensity (I) Candela cd 3/13/2020 2 United States Customary System of Units, USCS • The USCS was originally used in Great Britain, but it is today primarily used in the US. Why does the US stand out against SI? The reasons are complex and involve economics, logistics and culture. • One of the major distinctions between the SI and USCS is that mass is a base quantity in the SI, whereas force is a base quantity in the USCS. Unit of force in USCS is ‘ pound-force’ with the abbreviation lbf. It is common to use the shorter terminology ‘pound’ and the abbreviation lb. • Another distinction is that the USCS employs two different dimensions for mass; pound-mass, lbm and the slug (no abbreviation for the slug) (for calculations involving motion, gravitation, momentum, kinetic energy and acceleration the slug is preferred unit. -

Nature [February 18, 1897
J66 NATURE [FEBRUARY 18, 1897 The object of writing m for wfg, or of changing to the abso As it admits of a simple physical enunciation and a simple lute unit of force and writing m for w, is merely to get rid of g geometrical proof, I think it may interest your readers. in the dynamical equations, which concern the problems which A thin hollow cylinder with a uniform circular shell is alone are capable of direct human measurement. submerged in a fluid, and is lying with its axis horizontal. But this quantity g, so treacherous as Dr- Lodge can testify, The fluid is excluded from the cylinder by smooth face-plates should always be kept carefully in sight ; any attempt to get rid of the same density as the fluid. The weight of the shell of it merely causes it to reappear elsewhere in an unexpected is such that the cylinder displaces its own weight of the fluid. place (expe!les jurea, &c.). It is evident, then, that the cylinder, as a whole, is in neutral The engineer can be left to take care of himself, and does not equilibrium, at any depth below the surface. But, further, if require to be instructed in an art with which he is perfectly the shell be supposed to be perfectly flexible and incompressible, familiar. Considering that he has been compelled to create for it is still in equilibrium under its own weight and the fluid himself, without professorial assistance, the whole theory of the pressure. internal stresses of rapidly reciprocating machinery as causing Let the figure represent a ring of the cylinder one foot long, vibration, it cannot be correct to say that acceleration does not normal to the paper, and let the unit of weight be that of a come under his notice. -

SI Metric Units Vs USA Measures
Modern metric system (SI) units Some of the old pre-metric measures and old metric units still in use in the USA. This not a complete list — there are many others. acre (commercial) (36 000 ft2), acre (US survey) (43 560 ft2), acre (US international foot) (43 560 ft2), acre foot (43 560 ft3), Almost all measurements in your life can be acre foot per day, acre foot per year, acre inch (3630 ft3), agate (1/14 inch), arc second, atmosphere, atmosphere (standard), done with the metric units that will fit on the bag (1/6 US dry bbl), bag (3 UK bushells), barleycorn, barrel (oil), barrel (petroleum), barrel (US cranberry), barrel (US dry), barrel (US federal proof spirits), barrel (US federal), barrel (US liquid), bbl, bbl (US fed), board foot, bottle (spirits), bottle back of a business card: (wine), British thermal unit, British thermal unit (32 °F), British thermal unit (68 °F), British thermal unit (98 °F), British thermal unit (International steam table), British thermal unit (International), British thermal unit (IT), British thermal unit (mean), British thermal unit (thermochemical), BThU, BTU, Btu (International table), Btu (mean), Btu (th), Btu/hour, bushel, bushel (barley) (USDA), bushel (oats) (USDA), bushel (rye) (USDA), bushel (shelled corn) (USDA), bushel (soybeans), bushel (US volume), bushel (wheat), bushel potatoes (USDA), cable (US), cal (15 °C), cal (20 °C), cal (4 °C), cal (IT), cal (mean), cal (th), caliber 1000 grams = 1 kilogram (US), calibre (UK), calorie, Calorie, calorie (16 °C), calorie (20 °C), calorie (4 °C), calorie -
The SI Metric Systeld of Units and SPE METRIC STANDARD
The SI Metric SystelD of Units and SPE METRIC STANDARD Society of Petroleum Engineers The SI Metric System of Units and SPE METRIC STANDARD Society of Petroleum Engineers Adopted for use as a voluntary standard by the SPE Board of Directors, June 1982. Contents Preface . ..... .... ......,. ............. .. .... ........ ... .. ... 2 Part 1: SI - The International System of Units . .. .. .. .. .. .. .. .. ... 2 Introduction. .. .. .. .. .. .. .. .. .. .. .. .. 2 SI Units and Unit Symbols. .. .. .. .. .. .. .. .. .. .. .. 2 Application of the Metric System. .. .. .. .. .. .. .. .. .. .. .. .. 3 Rules for Conversion and Rounding. .. .. .. .. .. .. .. .. .. .. .. .. 5 Special Terms and Quantities Involving Mass and Amount of Substance. .. 7 Mental Guides for Using Metric Units. .. .. .. .. .. .. .. .. .. .. .. .. .. 8 Appendix A (Terminology).. .. .. .. .. .. .. .. .. .. .. .. .. 8 Appendix B (SI Units). .. .. .. .. .. .. .. .. .. .. .. .. 9 Appendix C (Style Guide for Metric Usage) ............ ...... ..... .......... 11 Appendix D (General Conversion Factors) ................... ... ........ .. 14 Appendix E (Tables 1.8 and 1.9) ......................................... 20 Part 2: Discussion of Metric Unit Standards. .. .. .. .. .. .. .. .. 21 Introduction.. .. .. .. .. .. .. .. .. .. .. .. 21 Review of Selected Units. .. .. .. .. .. .. .. .. .. .. 22 Unit Standards Under Discussion ......................................... 24 Notes for Table 2.2 .................................................... 25 Notes for Table 2.3 ................................................... -

Units and Dimensions
Appendix A Units and Dimensions It is essential to distinguish between units and dimensions. Broadly speaking, physical parameters have the separate properties of size and dimension. A unit is a, more or less arbitrarily defined, amount or quantity in terms of which a parameter is defined. A dimension represents the definition of an inherent property, independent of the system of units in which it is expressed. For example, the dimension mass expresses the amount of material of which a body is constructed, the distance between the wing tips (wingspan) of an aeroplane has the dimension length. The mass of a body can be expressed in kilograms or in pounds, the wingspan can be expressed in metres or in feet. Many systems of units exist, each of which with their own advantages and drawbacks. Throughout this book the internationally accepted dynamical system SI is used, except in a few places as specially noted. The Imperial set of units still plays an important roll in aviation, in particular in the United States. Fundamental dimensions and units Dimensions can be written in symbolic form by placing them between square brackets. There are four fundamental units in terms of which the dimensions of all other physical quantities may be expressed. Purely mechanical rela- tionships are derived in terms of mass [M], length [L], and time [T]; thermo- dynamical relationships contain the temperature [θ] as well. A fundamental equation governing dynamical systems is derived from Newton’s second law of motion. This states that an external force F acting on a body is proportional to the product of its mass m and the acceleration a produced by the force: F = kF ma. -

International System of Units (SI Units) SI Units Structure of SI and JIS Z 8203
Contact Details Internet Before using the product, please check the http://www.daikinpmc.com/en/ guide pages at the front of this catalog. For latest information, PDF catalogs and operation manuals International System of Units (SI units) SI units Structure of SI and JIS Z 8203 The International System of Units, or SI, comprises seven ⎧ Base units (seven) | base units, prefixes to represent integer powers of 10 in the | 18 −18 ⎧ SI Supplementary units (two) range from 10 to −10 , supplementary units of radians Unit ⎨ | | ⎧Derived units with special names and steradians, and twenty-seven derived units defined | | ⎧ | Derived units (eighteen) SI ⎨ ⎩ ⎨ according to the resolution by the International Committee | | | | Other derived units | ⎩ of Weights and Measures. JIS Z 8203 ⎨ Prefixes (sixteen) and integer powers of 10 of SI units | ⎩ It is often abbreviated as SI from its French expression | "Systeme International d'Unites". ⎩ Important units outside SI - Usable in parallel with SI units Its use in ISO standards started in 1971. In Japan, the Conference on Standardization of the Japanese Industrial Standards Committee decided in 1972 to introduce the SI units to JIS in a stepwise manner. And in 1974, JIS Z 8203 International System of Units (SI) (established based Appendix table 4 Example of SI derived units on ISO1000) and the guide for its use were enacted and that are expressed with special names promulgated, and their dissemination is in progress. Quantity Name Symbol viscosity pascal second Pa·s Appendix table 1 SI base units moment