Policy: 201101 Mrx Initial Effective Date: 02/17/2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actemra® (Tocilizumab)
Actemra® (tocilizumab) (Intravenous) Document Number: MODA-0002 Last Review Date: 10/26/2020 Date of Origin: 09/21/2010 Dates Reviewed: 12/2010, 03/2011, 05/2011, 06/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 09/2012, 11/2012, 12/2012, 03/2013, 06/2013, 09/2013, 11/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 05/2015, 09/2015, 12/0215, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 05/2017, 09/2017, 12/2017, 03/2018, 06/2018, 10/2018, 10/2019, 10/2020, 11/2020 I. Length of Authorization Coverage will be provided as follows: o Castleman’s Disease: 4 months and may be renewed o Cytokine Release Syndrome: 4 doses only and may not be renewed o Immune Checkpoint Inhibitor related arthritis: 1 dose and may not be renewed o All other indications: 6 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: o Actemra 80 mg/4 mL vial: 1 vial per 14 days o Actemra 200 mg/10 mL vial: 1 vial per 14 days o Actemra 400 mg/20 mL vial: 2 vials per 14 days B. Max Units (per dose and over time) [HCPCS Unit]: Diagnosis Billable Units Interval (days) Rheumatoid Arthritis & Polyarticular Juvenile Idiopathic 800 28 Arthritis, NMOSD Systemic Juvenile Idiopathic Arthritis, Castleman’s Disease (NHL) & Acute Graft Versus Host Disease 800 14 (aGVHD) Cytokine Release Syndrome (CRS) 3200 1 course of therapy only Immune Checkpoint Inhibitor related arthritis 800 1 course of therapy only III. -

Actemra (Tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA
Policy Title: Actemra (tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA Effective Date: 01/01/2020 Review Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Revision Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Purpose: To support safe, effective and appropriate use of Actemra (tocilizumab). Scope: Medicaid, Commerical, Medicare-Medicaid Plan (MMP) Policy Statement: Actemra (tocilizumab) is covered under the Medical Benefit when used within the following guidelines for non-oncology indications. Use outside of these guidelines may result in non-payment unless approved under an exception process. For oncology indications, please refer to NHPRI Oncology Policy Procedure: Coverage of Actemra (tocilizumab) will be reviewed prospectively via the prior authorization process based on criteria below. Initial Criteria: Patient has been evaluated and screened for the presence of latent TB infection prior to initiating treatment; AND Patient does not have an active infection, including clinically important localized infections; AND Must not be administered concurrently with live vaccines; AND Patient is not on concurrent treatment with another TNF-inhibitor, biologic response modifier or other non-biologic agent (i.e., apremilast, tofacitinib, baricitinib); MMP members who have previously received this medication within the past 365 days are not subject to Step Therapy Requirements Rheumatoid Arthritis Patient is 18 years or older; AND Physician has assessed baseline disease severity utilizing an objective measure/tool; AND -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -
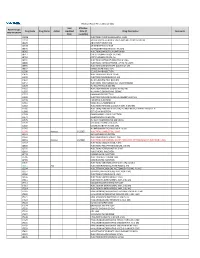
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients With
433 A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients with High-Risk Cutaneous Squamous Cell Carcinoma (CSCC) Danny Rischin,1 Matthew G. Fury,2 Israel Lowy,2 Elizabeth Stankevich,3 Siyu Li,2 Hyunsil Han,2 Sandro V. Porceddu4 1Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia; 2Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; 3Regeneron Pharmaceuticals, Inc., Basking Ridge, NJ, USA; 4School of Medicine, University of Queensland, Herston, Queensland, Australia; Department of Radiation Oncology, Princess Alexandra Hospital, Woolloongabba, Queensland, Australia. Background Figure 1. C-POST study design Table 2. Key exclusion criteria • Squamous cell carcinoma arising from non-cutaneous sites Summary Cutaneous squamous cell carcinoma (CSCC) Patients with high-risk CSCC often experience relapse with Surgery, with high-risk • Concurrent malignancy other than localized CSCC and/or history of • CSCC is the second most common skin cancer with an estimated features on surgical locoregional recurrence or distant metastases despite initial malignancy other than localized CSCC within 3 years of date of • 1 pathology report incidence of around 1 million cases per year in the US. Worldwide, randomization, except for tumors with negligible risk of metastasis treatment with surgery and post-operative radiation. reports show an annual rise in incidence of 3–7% in most countries.2 350 mg cemiplimab Optional cemiplimab re-treatment after disease recurrence -

NICE UPDATE for COMMISSIONERS April 2019
South, Central and West NICE UPDATE FOR COMMISSIONERS April 2019 This NICE Update for Commissioners includes: At-a-glance summary Headline update: what’s been published? Guidance and quality standards published by NICE in March 2019 What’s new for CCGs? Horizon scanning What’s coming out from NICE in the next six months? For your reference, a summary of the types of NICE guidance Reference – a guide to NICE products The next (May 2019) NICE Update for Commissioners will be issued at the beginning of June 2019. For further information about NICE guidance and its implementation contact: Tiina Korhonen, Clinical Effectiveness Lead Kathryn Markey, Clinical Effectiveness Manager Kate Forbes, Clinical Effectiveness Manager Rebecca Hodge, Clinical Effectiveness Manager Gill Barlow, Clinical Effectiveness Manager Katie Newens, Clinical Effectiveness Researcher Rachel Finch, Clinical Effectiveness Administrator [email protected] 1 | p a g e At-a-glance summary The table below shows ALL NICE guidance published in April2019. Those likely to have significant impact for CCG commissioners are discussed further in the ‘What’s new for Clinical Commissioning Groups’ section (link to relevant section provided within guidance reference). Guidance type and Title Commissioner(s) Main providers(s) Impact for CCG commissioners (financial /public reference interest/quality of care) Technology Appraisal – Daratumumab with NHS England Secondary care - TA573 bortezomib and acute and dexamethasone for Tertiary care previously treated multiple myeloma Technology Appraisal – Certolizumab pegol for CCGs Primary care, NICE does not expect this guidance to have a TA574 treating moderate to severe secondary care - significant impact on resources; that is, it will be plaque psoriasis acute and tertiary less than £5 million per year in England (or care £9,100 per 100,000 population) because the technology is an option alongside current standard treatment options and is available at a similar price. -

Libtayo® (Cemiplimab-Rwlc)
Libtayo® (cemiplimab-rwlc) (Intravenous) Document Number: IC-0398 Last Review Date: 06/01/2021 Date of Origin: 10/30/2018 Dates Reviewed: 11/2018, 03/2019, 06/2019, 09/2019, 12/2019, 03/2020, 6/2020, 12/2020, 03/2021, 06/2021 I. Length of Authorization Coverage will be provided for six months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: • Libtayo 350 mg/7 mL single-use vial: 1 vial per 21 days B. Max Units (per dose and over time) [HCPCS Unit]: • 350 billable units every 21 days III. Initial Approval Criteria1 Coverage is provided for the following conditions: • Patient is at least 18 years of age; AND Universal Criteria 1 • Patient has not received previous therapy with a programmed death (PD-1/PD-L1)-directed therapy (e.g., avelumab, pembrolizumab, atezolizumab, durvalumab, nivolumab, dostarlimab, etc.), unless otherwise specified; AND • Used as a single-agent therapy; AND • Patient has not received previous therapy with a cytotoxic T-lymphocyte antigen 4 (CTLA-4) targeting agent (e.g., ipilimumab, etc.) within the 4 weeks prior to therapy; AND Cutaneous Squamous Cell Carcinoma (CSCC) † 1-5 • Patient has nodal or distant metastatic disease, locally advanced disease, inoperable or not fully resectable regional disease, or regional recurrence; AND • Patient is not a candidate for curative surgery or curative radiation therapy Basal Cell Carcinoma (BCC) † 1,2,6 • Patient has locally advanced OR nodal, regional, or distant metastatic disease; AND Proprietary & Confidential © 2021 Magellan Health, -

Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read
Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read: This document contains information about the drugs we cover when they are administered to you in a Participating Medical Office. What is the Kaiser Permanente Northwest Medical Benefit Formulary? A formulary is a list of covered drugs chosen by a group of Kaiser Permanente physicians and pharmacists known as the Formulary and Therapeutics Committee. This committee meets regularly to evaluate and select the safest, most effective medications for our members. Kaiser Permanente Formulary The formulary list that begins on the next page provides information about some of the drugs covered by our plan when they are administered to you in a Participating Medical Office. Depending on your medical benefits, you may pay a cost share for the drug itself. The first column of the chart lists the drug’s generic name. The second column lists the brand name. Most administered medications and vaccines are only available as brand name drugs. Contraceptive Drugs and Devices Your provider may prescribe as medically necessary any FDA-approved contraceptive drug or device, including those on this formulary, which you will receive at no cost share. Last Updated 09/01/2021 Generic Name Brand Name Clinic Administered Medications (ADMD) ABATACEPT ORENCIA ABOBOTULINUM TOXIN A DYSPORT ADALIMUMAB HUMIRA ADO-TRASTUZUMAB EMTANSINE KADCYLA AFAMELANOTIDE ACETATE SCENESSE AFLIBERCEPT EYLEA AGALSIDASE BETA FABRAZYME ALDESLEUKIN PROLEUKIN ALEMTUZUMAB LEMTRADA ALGLUCOSIDASE ALFA LUMIZYME ARALAST, GLASSIA, -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

CDER Therapeutic Biologic Products List
CDER Therapeutic Biologic Products This list is intended to include all the Center for Drug Evaluation and Research (CDER) user fee billable therapeutic biological products and potencies approved under Section 351 of the Public Health Service Act. The Orange Book includes a section entitled "Drug Products with Approval under Section 505 of the Act Administered by CBER." Included on that list are several products that have been transferred to CDER which would be considered billable also. Program fees are assessed for each potency in which the approved (non-revoked, non-suspended) product is manufactured in final dosage form. When evaluating the specific strength or potency of a drug in final dosage form for purposes of assessing program fees for liquid parenteral biological products, CDER intends to take into consideration both the total amount of drug substance in mass or units of activity in a product and the concentration of drug substance (mass or units of activity per unit volume of product). Biologic products considered to have a different strength or potency in a final dosage form will be given separate entries in the Biologics List and assessed separate program fees. An auto-injector that has the same strength or potency as a prefilled syringe or vial will generally be assessed a separate prescription drug program fee. In certain circumstances, products which have been discontinued from marketing but are still licensed are not assessed program fees. Those products are identified on the CDER Discontinued Biologic Product List section. The potency information contained in this list is based on information in our database. -

Avelumab) Name
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: 761049Orig1s009 Trade Name: Bavencio injection, for intravenous use Generic or Proper (avelumab) Name: Sponsor: EMD Serono Approval Date: June 30, 2020 Indication: For the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed with first-line platinum-containing chemotherapy. CENTER FOR DRUG EVALUATION AND RESEARCH 761049Orig1s009 CONTENTS Reviews / Information Included in this NDA Review. Approval Letter X Other Action Letters Labeling X REMS Officer/Employee List Multidiscipline Review(s) X • Summary Review • Office Director • Cross Discipline Team Leader • Clinical • Non-Clinical • Statistical • Clinical Pharmacology • Clinical Microbiology/Virology Product Quality Review(s) X Other Reviews X Risk Assessment and Risk Mitigation Review(s) Proprietary Name Review(s) Administrative/Correspondence Document(s) CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761049Orig1s009 APPROVAL LETTER BLA 761049/S-009 SUPPLEMENT APPROVAL/ FULFILLMENT OF POSTMARKETING REQUIREMENT EMD Serono, Inc. Attention: Jennifer L. Stevens, JD Executive Director US Hub Lead/Global Regulatory Program Lead 45A Middlesex Turnpike Billerica, MA 01821 Dear Ms. Stevens: Please refer to your supplemental biologics license application dated April 7, 2020, received April 7, 2020, and your amendments, submitted under section 351(a) of the Public Health Service Act for Bavencio (avelumab) Injection. This Prior Approval supplemental biologics application provides for a new indication for the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed with first-line platinum-containing chemotherapy. We also refer to your biologics license application (BLA) 761078, approved May 9, 2017, under the regulations at 21 CFR 601 Subpart E for Accelerated Approval of Biological Products for Serious or Life-Threatening Illnesses. -

Developmental Therapeutics Immunotherapy
DEVELOPMENTAL THERAPEUTICS—IMMUNOTHERAPY 2500 Oral Abstract Session Clinical activity of systemic VSV-IFNb-NIS oncolytic virotherapy in patients with relapsed refractory T-cell lymphoma. Joselle Cook, Kah Whye Peng, Susan Michelle Geyer, Brenda F. Ginos, Amylou C. Dueck, Nandakumar Packiriswamy, Lianwen Zhang, Beth Brunton, Baskar Balakrishnan, Thomas E. Witzig, Stephen M Broski, Mrinal Patnaik, Francis Buadi, Angela Dispenzieri, Morie A. Gertz, Leif P. Bergsagel, S. Vincent Rajkumar, Shaji Kumar, Stephen J. Russell, Martha Lacy; Mayo Clinic, Rochester, MN; The Ohio State University, Columbus, OH; Mayo Clinic, Scottsdale, AZ; Division of Hematology, Mayo Clinic, Roches- ter, MN; Vyriad and Mayo Clinic, Rochester, MN Background: Oncolytic virotherapy is a novel immunomodulatory therapeutic approach for relapsed re- fractory hematologic malignancies. The Indiana strain of Vesicular Stomatitis Virus was engineered to encode interferon beta (IFNb) and sodium iodine symporter (NIS) to produce VSV-IFNb-NIS. Virally en- coded IFNb serves as an index of viral proliferation and enhances host anti-tumor immunity. NIS was in- serted to noninvasively assess viral biodistribution using SPECT/PET imaging. We present the results of the phase 1 clinical trial NCT03017820 of systemic administration of VSV-IFNb-NIS among patients (pts) with relapsed refractory Multiple Myeloma (MM), T cell Lymphoma (TCL) and Acute myeloid Leu- 9 kemia (AML). Methods: VSV-IFNb-NIS was administered at 5x10 TCID50 (50% tissue culture infec- 11 tious dose) dose level 1 to dose level 4, 1.7x10 TCID50. The primary objective was to determine the maximum tolerated dose of VSV-IFNb-NIS as a single agent. Secondary objectives were determination of safety profile and preliminary efficacy of VSV-IFNb-NIS.