Evolution of Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microchiroptera: Mystacinidae) from Australia, with a Revised Diagnosis of the Genus
New Miocene Icarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus SUZANNE HAND, MICHAEL ARCHER & HENK GODTHELP HAND, S.l., ARCHER, M. & GODTHELP, H., 2001:12:20. New Miocene lcarops material (Microchiroptera: Mystacinidae) from Australia, with a revised diagnosis of the genus. Memoirs of the Association of Australasian Palaeontologists 25,139-146. ISSN 0810-8889 New fossil material referable to Icarops paradox Hand et al., 1998 is described from the early Miocene Judith's Horizontalis Site in the Riversleigh World Heritage Property of northwestern Queensland. Fused dentaries contain the partial lower dentition of I. paradox. The diagnosis of the genus Icarops is revised. The new material confirms the identity of Icarops species as mystacinids and enablesre-examination of interrelationships between extinct and extant members of this Gondwanan bat family. S.J: Hand, M. Archer* & H. Godthelp, School of Biological Science, University of New South Wales,New South Wales, 2052; * also Australian Museum, 6-8 College St, Sydney, New South Wales,2000. Received ]4 December 2000 Keywords: Mystacinidae, Icarops, Mystacina, bat, lower dentition, Miocene, Riversleigh THE FIRST pre-Pleistocene record for the QMF refers to specimens held in the fossil Mystacinidae and first record of this bat family collections of the QueenslandMuseum, Brisbane. from outside New Zealand were reported by Hand et al. ( 1998) from Miocene sedimentsin Australia. SYSTEMAllC PALAEONTOLOGY Three species of the new mystacinid genus Icarops were described: Icarops breviceps from OrderCIllROPTERAB1wnenbach, 1779 the middle Miocene Bullock Creek deposit of the SuborderMICROCIllROPTERA Dobson, 1875 Northern Territory; I. aenae from the early SuperfamilyNocmIoNoIDEA Van Va1en, Miocene Wayne's Wok deposit, D Site Plateau, 1979 Riversleigh, northwestern Queensland; and I. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Pacific Sheath-Tailed Bat American Samoa Emballonura Semicaudata Semicaudata Species Report April 2020
Pacific Sheath-tailed Bat American Samoa Emballonura semicaudata semicaudata Species Report April 2020 U.S. Fish and Wildlife Service Pacific Islands Fish and Wildlife Office Honolulu, HI Cover Photo Credits Shawn Thomas, Bat Conservation International. Suggested Citation USFWS. 2020. Species Status Assessment for the Pacific Sheath Tailed Bat (Emballonura semicaudata semicaudata). April 2020 (Version 1.1). U.S. Fish and Wildlife Service, Pacific Islands Fish and Wildlife Office, Honolulu, HI. 57 pp. Primary Authors Version 1.1 of this document was prepared by Mari Reeves, Fred Amidon, and James Kwon of the Pacific Islands Fish and Wildlife Office, Honolulu, Hawaii. Preparation and review was conducted by Gregory Koob, Megan Laut, and Stephen E. Miller of the Pacific Islands Fish and Wildlife Office. Acknowledgements We thank the following individuals for their contribution to this work: Marcos Gorresen, Adam Miles, Jorge Palmeirim, Dave Waldien, Dick Watling, and Gary Wiles. ii Executive Summary This Species Report uses the best available scientific and commercial information to assess the status of the semicaudata subspecies of the Pacific sheath-tailed bat, Emballonura semicaudata semicaudata. This subspecies is found in southern Polynesia, eastern Melanesia, and Micronesia. Three additional subspecies of E. semicaudata (E.s. rotensis, E.s. palauensis, and E.s. sulcata) are not discussed here unless they are used to support assumptions about E.s. semicaudata, or to fill in data gaps in this analysis. The Pacific sheath-tailed bat is an Old-World bat in the family Emballonuridae, and is found in parts of Polynesia, eastern Melanesia, and Micronesia. It is the only insectivorous bat recorded from much of this area. -

Timeline of the Evolutionary History of Life
Timeline of the evolutionary history of life This timeline of the evolutionary history of life represents the current scientific theory Life timeline Ice Ages outlining the major events during the 0 — Primates Quater nary Flowers ←Earliest apes development of life on planet Earth. In P Birds h Mammals – Plants Dinosaurs biology, evolution is any change across Karo o a n ← Andean Tetrapoda successive generations in the heritable -50 0 — e Arthropods Molluscs r ←Cambrian explosion characteristics of biological populations. o ← Cryoge nian Ediacara biota – z ← Evolutionary processes give rise to diversity o Earliest animals ←Earliest plants at every level of biological organization, i Multicellular -1000 — c from kingdoms to species, and individual life ←Sexual reproduction organisms and molecules, such as DNA and – P proteins. The similarities between all present r -1500 — o day organisms indicate the presence of a t – e common ancestor from which all known r Eukaryotes o species, living and extinct, have diverged -2000 — z o through the process of evolution. More than i Huron ian – c 99 percent of all species, amounting to over ←Oxygen crisis [1] five billion species, that ever lived on -2500 — ←Atmospheric oxygen Earth are estimated to be extinct.[2][3] Estimates on the number of Earth's current – Photosynthesis Pong ola species range from 10 million to 14 -3000 — A million,[4] of which about 1.2 million have r c been documented and over 86 percent have – h [5] e not yet been described. However, a May a -3500 — n ←Earliest oxygen 2016 -
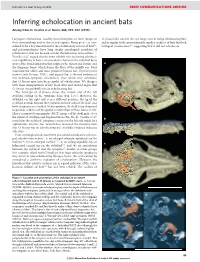
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Cormura Brevirostris. by Enrico Bernard Published 18 December 2003 by the American Society of Mammalogists
MAMMALIAN SPECIES No. 737, pp. 1±3, 3 ®gs. Cormura brevirostris. By Enrico Bernard Published 18 December 2003 by the American Society of Mammalogists Cormura Peters, 1867 from same locality are: zygomatic breadth, 10.02 (9.64±10.36), 10.03 (9.85±10.16); length of maxillary toothrow, 6.17 (6.01±6.35), Emballonura Wagner, 1843:367. Type species Emballonura bre- 6.18 (5.91±6.50); breadth across molars, 7.27 (6.98±7.55), 7.39 virostris Wagner. (7.23±7.55); condylocanine length for males and females combined, Cormura Peters, 1867:475. Based on Emballonura brevirostris 14.23 (13.67±14.73). Ranges of external and skull measurements Wagner. (sample sizes in parentheses) from Suriname of sexes combined Myropteryx Miller, 1906:59±60. Type species Myropteryx pullus (Husson 1962) are: length of forearm, 41.5±47.0 (10); length of 3rd Miller. digit, metacarpal, 40.0±42.0 (10); length of 3rd digit, 1st phalanx, 12.0±14.0 (10); length of 3rd digit, 2nd phalanx, 19.0±22.0 (10); CONTEXT AND CONTENT. Order Chiroptera, suborder length of 4th digit, metacarpal, 33.0±36.0 (10); length of 4th digit, Microchiroptera, family Emballonuridae, subfamily Emballonuri- 1st phalanx, 9.0±10.5 (10); length of 4th digit, 2nd phalanx, 6.0± nae. Cormura is monotypic. 8.0 (9); length of 5th digit, metacarpal, 30.5±34.0 (10); length of 5th digit, 1st phalanx, 11.0±11.5 (10); length of 5th digit, 2nd phalanx, 5.0±7.0 (9); length of tibia, 15.0±16.5 (10); length of hind Cormura brevirostris (Wagner, 1843) foot, 6.5±7.0 (9); length of calcar, 13.0±15.5 (9); greatest length of Wagner's Sac-winged Bat skull, 15.1±15.8 (4); condylobasal length, 14.7 (1); condyle to front of canine, 13.6±14.3 (5); basal length, 11.3±13.0 (4); palatal length, Emballonura brevirostris Wagner, 1843:367. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Rapid and Early Post-Flood Mammalian Diversification Videncede in the Green River Formation
The Proceedings of the International Conference on Creationism Volume 6 Print Reference: Pages 449-457 Article 36 2008 Rapid and Early Post-Flood Mammalian Diversification videncedE in the Green River Formation John H. Whitmore Cedarville University Kurt P. Wise Southern Baptist Theological Seminary Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Whitmore, John H. and Wise, Kurt P. (2008) "Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation," The Proceedings of the International Conference on Creationism: Vol. 6 , Article 36. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol6/iss1/36 In A. A. Snelling (Ed.) (2008). Proceedings of the Sixth International Conference on Creationism (pp. 449–457). Pittsburgh, PA: Creation Science Fellowship and Dallas, TX: Institute for Creation Research. Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation John H. Whitmore, Ph.D., Cedarville University, 251 N. Main Street, Cedarville, OH 45314 Kurt P. Wise, Ph.D., Southern Baptist Theological Seminary, 2825 Lexington Road. -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34.