Microchiroptera: Mystacinidae) from Australia, with a Revised Diagnosis of the Genus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Mammals 61
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORCHAPTER SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Classification © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 4 NOT FORof SALE MammalsOR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION. 2ND PAGES 9781284032093_CH04_0060.indd 60 8/28/13 12:08 PM CHAPTER 4: Classification of Mammals 61 © Jones Despite& Bartlett their Learning,remarkable success, LLC mammals are much less© Jones stress & onBartlett the taxonomic Learning, aspect LLCof mammalogy, but rather as diverse than are most invertebrate groups. This is probably an attempt to provide students with sufficient information NOT FOR SALE OR DISTRIBUTION NOT FORattributable SALE OR to theirDISTRIBUTION far greater individual size, to the high on the various kinds of mammals to make the subsequent energy requirements of endothermy, and thus to the inabil- discussions of mammalian biology meaningful. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
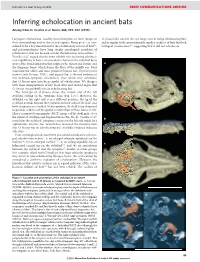
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Mystacina Tuberculata) Through a Pest Control Operation Using the Toxin Pindone in Bait Stations
AvailableO’Donnell on-line et al.: at:Bat http://www.newzealandecology.org/nzje/ survival after pindone operation 291 SHORT COMMUNICATION Survival of PIT-tagged lesser short-tailed bats (Mystacina tuberculata) through a pest control operation using the toxin pindone in bait stations Colin F.J. O’Donnell1*, Hannah Edmonds2 and Joanne M. Hoare1 1Research and Development Group, Department of Conservation, PO Box 13049, Christchurch 8141, New Zealand 2Department of Conservation, PO Box 29, Te Anau 9640, New Zealand *Author for correspondence (Email: [email protected]) Published on-line: 21 March 2011 Abstract: Introduced mammalian predators are a major threat to New Zealand’s wildlife, including bats. Controlling these predators using traps and poison baits can reduce their impact on bat populations. However, lesser short-tailed bats (Mystacina tuberculata) are potentially susceptible to toxins used for pest control in New Zealand forests because of their broad diet and habit of feeding on the ground. Therefore, the risk of secondary poisoning should always be assessed before new toxins are used in areas inhabited by lesser short- tailed bats. We measured survivorship of a sample of lesser short-tailed bats monitored before, during and after deployment of the first-generation anticoagulant toxin pindone in the Eglinton Valley, Fiordland, during late winter and summer 2009−2010. Pindone-laced cereal baits were deployed in bait stations from September to late December 2009 in an effort to control rats (Rattus spp.). Communal roosts of the one lesser short-tailed bat colony in the valley are located entirely within or immediately adjacent to the area poisoned. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Discovery Place Nature Merit Badge Workshops 2018
1 Discovery Place Nature formerly, Charlotte Nature Museum, a division of Discovery Place, 1658 Sterling Rd, Charlotte, NC 28209 Merit Badge Workshops 2018 https://nature.discoveryplace.org/programs-and-classes/scout-programs All Merit Badges are scheduled for 9:00 am to 4:00 pm and will meet in the Naturalist Lab at Discovery Place Nature. Date and Time Merit Badge Workshop Saturday, January 13, 9:00 am to 4:00 pm Astronomy* Saturday, February 10, 9:00 am to 4:00 pm Astronomy* Saturday, March 10, 9:00 am to 4:00 pm Environmental Science Saturday, March 24, 9:00 am to 4:00 pm Geocaching* Saturday, April 14, 9:00 am to 4:00 pm Nature* Saturday, May 12, 9:00 am to 4:00 pm Mammal Study Saturday, June 9, 9:00 am to 4:00 pm Environmental Science July NO MERIT BADGES Saturday, August 11, 9:00 am to 4:00 pm Insect Study Saturday, August 25, 9:00 am to 4:00 pm Reptile and Amphibian Study* Saturday, September 8, 9:00 am to 4:00 pm Bird Study Saturday, October 13, 9:00 am to 4:00 pm Environmental Science Saturday, November 10, 9:00 am to 4:00 pm Astronomy* Saturday, December 8 , 9:00 am to 4:00 pm Environmental Science *Astronomy, Geocaching, Nature, and Reptile and Amphibian Study are workshops for which Scouts can qualify for partial completion. Additional homework is required to complete the requirements for these merit badges. Other merit badge workshops offer opportunity for completions IF Scouts complete pre-course homework and present the homework to the Merit Badge Counselor when the course meets. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Rapid and Early Post-Flood Mammalian Diversification Videncede in the Green River Formation
The Proceedings of the International Conference on Creationism Volume 6 Print Reference: Pages 449-457 Article 36 2008 Rapid and Early Post-Flood Mammalian Diversification videncedE in the Green River Formation John H. Whitmore Cedarville University Kurt P. Wise Southern Baptist Theological Seminary Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Whitmore, John H. and Wise, Kurt P. (2008) "Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation," The Proceedings of the International Conference on Creationism: Vol. 6 , Article 36. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol6/iss1/36 In A. A. Snelling (Ed.) (2008). Proceedings of the Sixth International Conference on Creationism (pp. 449–457). Pittsburgh, PA: Creation Science Fellowship and Dallas, TX: Institute for Creation Research. Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation John H. Whitmore, Ph.D., Cedarville University, 251 N. Main Street, Cedarville, OH 45314 Kurt P. Wise, Ph.D., Southern Baptist Theological Seminary, 2825 Lexington Road. -

How Many Species of Mammals Are There?
Journal of Mammalogy, 99(1):1–14, 2018 DOI:10.1093/jmammal/gyx147 INVITED PAPER How many species of mammals are there? CONNOR J. BURGIN,1 JOCELYN P. COLELLA,1 PHILIP L. KAHN, AND NATHAN S. UPHAM* Department of Biological Sciences, Boise State University, 1910 University Drive, Boise, ID 83725, USA (CJB) Department of Biology and Museum of Southwestern Biology, University of New Mexico, MSC03-2020, Albuquerque, NM 87131, USA (JPC) Museum of Vertebrate Zoology, University of California, Berkeley, CA 94720, USA (PLK) Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06511, USA (NSU) Integrative Research Center, Field Museum of Natural History, Chicago, IL 60605, USA (NSU) 1Co-first authors. * Correspondent: [email protected] Accurate taxonomy is central to the study of biological diversity, as it provides the needed evolutionary framework for taxon sampling and interpreting results. While the number of recognized species in the class Mammalia has increased through time, tabulation of those increases has relied on the sporadic release of revisionary compendia like the Mammal Species of the World (MSW) series. Here, we present the Mammal Diversity Database (MDD), a digital, publically accessible, and updateable list of all mammalian species, now available online: https://mammaldiversity.org. The MDD will continue to be updated as manuscripts describing new species and higher taxonomic changes are released. Starting from the baseline of the 3rd edition of MSW (MSW3), we performed a review of taxonomic changes published since 2004 and digitally linked species names to their original descriptions and subsequent revisionary articles in an interactive, hierarchical database. We found 6,495 species of currently recognized mammals (96 recently extinct, 6,399 extant), compared to 5,416 in MSW3 (75 extinct, 5,341 extant)—an increase of 1,079 species in about 13 years, including 11 species newly described as having gone extinct in the last 500 years. -

Bats NW Spring 08
Become a Bats Northwest Member Become a Bats Northwest Member. Join us in the adventure to learn more about our bat neighbors! BatsBats News Membership Options: $35 $50 $75 $100 Other $_________________ Name:_________________________________________________________________________________ Address:______________________________________________________________________________ Bats Northwest Mailing Address: NorthwestNorthwest P.O. Box 3026 _______________________________________________________________________________________ BNW IS A NON-PROFIT, ALL VOLUNTEER CONSERVATION ORGANIZATION SPRING 2008 Lynnwood, WA 98046 Phone:_________________________________________________________________________________ Bats and Humans: provide as much as 40% of the lift force that 206.256.0406 Email:_________________________________________________________________________________ helped the bats stay aloft. A Helping Hand Bats Northwest web site: Bats go a step further by using their embedded www.batsnorthwest.org by Kathleen Bander thumbs and fingers as flaps like those on an airplane wing, to alter the curve of the wing BATS NW T-SHIRTS What amazing adaptation do bats and insects and create the lift force needed to hover. have in common? No, it isn’t just that insects You’ll look great in our Bats Northwest Long-Sleeved T-Shirt! It also makes a are food for bats. Nor is it that flight is the main Scientists found that the reason bats are able wonderful gift. mode of transportation for both. to produce LEVs is because they can actively Heavyweight cotton, natural off-white, with a brightly colored bat graphic. change the curvature of their wings using their The commonality is the ability, only lately elongaged fingers and muscle fibers unlike I WOULD LIKE TO ORDER ____ (QUANTITY) BATS NORTHWEST LONG-SLEEVED T-SHIRT(S) AT $22.00 EACH FOR witnessed by scientists, of both bats and insects which rely on extreme speed. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations