The Evolution of Flight in Bats: a Novel Hypothesis Sophia C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Northwestwildlife.Com Species Reports
A publication by: NORTHWEST WILDLIFE PRESERVATION SOCIETY Northern Flying Squirrel Glaucomys sabrinus Photo credit: US Fish and Wildlife By Renee Picard There are two species of flying squirrels that live in North America. The northern flying squirrel (with 25 sub-species) may be found in forests throughout most of Canada, except for the central prairies and the extreme North; also in the U.S. in Alaska and northern areas of the Rockies and Appalachians. The southern flying squirrel (with 10 sub-species) inhabits a broad range in the eastern and midwestern United States, but in Canada is only found in very small, scattered pockets of southeastern Ontario. The southern species is considered ‘vulnerable’ but the northern species is not at risk. It is the northern squirrel that you would be likely to encounter in the Pacific Northwest, so it is the focus of this article. Characteristics The scientific name for the northern flying squirrel is Glaucomys sabrinus. Glaucos means for silver or grey, mys means mouse, and sabrinus come from the Latin word for river-nymph. So you will notice them often in riparian areas, near streams and rivers. Their colours range from tan to cinnamon and they have greyish-white belly fur. They are about 30 cm (12 in.) long and weigh about 139 g (46 oz.) Flying squirrels have big black eyes and this characteristic helps their night vision for they are nocturnal animals. You might be surprised to find that, despite their name, flying squirrels do not really fly—they glide down from branch to branch. The front and back legs are connected with a thin fold of furry skin or membrane called a patagium. -

Timeline of the Evolutionary History of Life
Timeline of the evolutionary history of life This timeline of the evolutionary history of life represents the current scientific theory Life timeline Ice Ages outlining the major events during the 0 — Primates Quater nary Flowers ←Earliest apes development of life on planet Earth. In P Birds h Mammals – Plants Dinosaurs biology, evolution is any change across Karo o a n ← Andean Tetrapoda successive generations in the heritable -50 0 — e Arthropods Molluscs r ←Cambrian explosion characteristics of biological populations. o ← Cryoge nian Ediacara biota – z ← Evolutionary processes give rise to diversity o Earliest animals ←Earliest plants at every level of biological organization, i Multicellular -1000 — c from kingdoms to species, and individual life ←Sexual reproduction organisms and molecules, such as DNA and – P proteins. The similarities between all present r -1500 — o day organisms indicate the presence of a t – e common ancestor from which all known r Eukaryotes o species, living and extinct, have diverged -2000 — z o through the process of evolution. More than i Huron ian – c 99 percent of all species, amounting to over ←Oxygen crisis [1] five billion species, that ever lived on -2500 — ←Atmospheric oxygen Earth are estimated to be extinct.[2][3] Estimates on the number of Earth's current – Photosynthesis Pong ola species range from 10 million to 14 -3000 — A million,[4] of which about 1.2 million have r c been documented and over 86 percent have – h [5] e not yet been described. However, a May a -3500 — n ←Earliest oxygen 2016 -
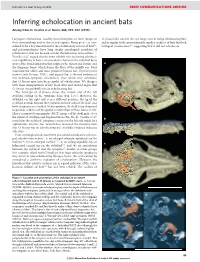
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam
viruses Article Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam Satoru Arai 1, Fuka Kikuchi 1,2, Saw Bawm 3 , Nguyễn Trường Sơn 4,5, Kyaw San Lin 6, Vương Tân Tú 4,5, Keita Aoki 1,7, Kimiyuki Tsuchiya 8, Keiko Tanaka-Taya 1, Shigeru Morikawa 9, Kazunori Oishi 1 and Richard Yanagihara 10,* 1 Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] (S.A.); [email protected] (F.K.); [email protected] (K.A.); [email protected] (K.T.-T.); [email protected] (K.O.) 2 Department of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 3 Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 4 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam; [email protected] (N.T.S.); [email protected] (V.T.T.) 5 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi, Vietnam 6 Department of Aquaculture and Aquatic Disease, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 7 Department of Liberal Arts, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 8 Laboratory of Bioresources, Applied Biology Co., Ltd., Tokyo 107-0062, Japan; [email protected] 9 Department of Veterinary Science, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] 10 Pacific Center for Emerging Infectious Diseases Research, John A. -

A Bird's Eye View of the Evolution of Avialan Flight
Chapter 12 Navigating Functional Landscapes: A Bird’s Eye View of the Evolution of Avialan Flight HANS C.E. LARSSON,1 T. ALEXANDER DECECCHI,2 MICHAEL B. HABIB3 ABSTRACT One of the major challenges in attempting to parse the ecological setting for the origin of flight in Pennaraptora is determining the minimal fluid and solid biomechanical limits of gliding and powered flight present in extant forms and how these minima can be inferred from the fossil record. This is most evident when we consider the fact that the flight apparatus in extant birds is a highly integrated system with redundancies and safety factors to permit robust performance even if one or more components of their flight system are outside their optimal range. These subsystem outliers may be due to other adaptive roles, ontogenetic trajectories, or injuries that are accommodated by a robust flight system. This means that many metrics commonly used to evaluate flight ability in extant birds are likely not going to be precise in delineating flight style, ability, and usage when applied to transitional taxa. Here we build upon existing work to create a functional landscape for flight behavior based on extant observations. The functional landscape is like an evolutionary adap- tive landscape in predicting where estimated biomechanically relevant values produce functional repertoires on the landscape. The landscape provides a quantitative evaluation of biomechanical optima, thus facilitating the testing of hypotheses for the origins of complex biomechanical func- tions. Here we develop this model to explore the functional capabilities of the earliest known avialans and their sister taxa. -

A Comparison of Flight Potential Among Small-Bodied Paravians
Chapter 11 High Flyer or High Fashion? A Comparison of Flight Potential among Small-Bodied Paravians T. ALEXANDER DECECCHI,1 HANS C.E. LARSSON,2 MICHAEL PITTMAN,3 AND MICHAEL B. HABIB4 ABSTRACT The origin of flight in birds and its relationship to bird origins itself has achieved something of a renaissance in recent years, driven by the discovery of a suite of small-bodied taxa with large pen- naceous feathers. As some of these specimens date back to the Middle Jurassic and predate the earli- est known birds, understanding how these potential aerofoil surfaces were used is of great importance to answering the question: which came first, the bird or the wing? Here we seek to address this question by directly comparing key members of three of the major clades of paravians: anchiorni- thines, Microraptor and Archaeopteryx across their known size classes to see how they differ in terms of major flight-related parameters (wing loading; disc loading; specific lift; glide speed; takeoff poten- tial). Using specimens with snout to vent length (SVL) ranging from around 150 mm to 400 mm and mass ranging from approximately 130 g to 2 kg, we investigated patterns of inter- and intraspe- cific changes in flight potential. We find that anchiornithines show much higher wing- and disc- loading values and correspondingly high required minimum glide and takeoff speeds, along with lower specific lift and flapping running outputs suggesting little to no flight capability in this clade. In contrast, we see good support for flight potential, either gliding or powered flight, for all size classes of both Microraptor and Archaeopteryx, though there are differing patterns of how this shifts ontogenetically. -

Jurnal Riset Veteriner Indonesia Journal of the Indonesian Veterinary Research P-ISSN: 2614-0187, E-ISSN:2615-2835 Volume 3 No
Jurnal Riset Veteriner Indonesia Journal of the Indonesian Veterinary Research P-ISSN: 2614-0187, E-ISSN:2615-2835 Volume 3 No. 1 (Januari 2019), pp. 1-9 journal.unhas.ac.id/index.php/jrvi/ This woks is licenced under a Creative Commons Attribution 4.0 International License. R eview Article Bats Oxidative Stress Defense (Pertahanan Stres Oksidatif pada Kelelawar) 1 1 1 Desrayni Hanadhita , Aryani Sismin Satjaningtyas , Srihadi Agungpriyono * 1Department of Anatomy Physiology and Pharmacology, Faculty of Veterinary Medicine, IPB University Jl. Agatis, Kampus IPB Dramaga, Bogor 16680, Indonesia *Corresponding authors: Srihadi Agungpriyono ([email protected]) Abstract Antioxidants and free radicals have long been known to be the main factors in the occurrence of degenerative diseases. Various studies related to antioxidants and free radicals which have implications for oxidative stress have increased in the last decade. Knowledge of stress oxidative physiology in various animals help in understanding the pathophysiology of diseases associated with oxidative stress. Bats are claimed to be the best known animals in term of survival compared to other mammals. Bats are reported to produce low reactive oxygen species (ROS) but high endogenous antioxidants that can prevent oxidative stress. Bats high defense against oxidative stress has implications for their extreme longevity, the role as a reservoir of viruses, and the potential as experimental animals. Key words: aging, animal model, antioxidant, comparative physiology, free radical Copyright © 2019 JRVI. All rights reserved. Introduction Oxidative stress has been a scourge for the health world for more than three decades (Sies, 2015). Research on oxidative stress status related to disease pathogenesis is increasing every year. -

Insectivores, Are Very Sharp to Bite Through the Exoskeleton of Insects Or the Skin of Fruit
Bats are mammals of the order Chiroptera, which means handwing. Bat’s forelimbs form webbed wings, making them the only mammals capable of true and sustained flight. Bats do not flap their entire forelimbs, like birds, but instead flap their spread-out digits, which are very long and covered with a thin membrane (patagium). Bats are present throughout most of the world in most temperate and tropical regions of both hemispheres, performing vital ecological roles of pollinating flowers and dispersing fruit seeds. It also known that bats eat insects and other pests and do not normally pose a threat to humans. They represent about 20% of all classified mammal species worldwide, with about 1,000 bat species. The order Chiroptera is divided into two suborders: Megachiroptera and Microchiroptera. Members of Megachiroptera are commonly referred to as flying foxes because of their fox-like faces. They are less specialized than Microhiroptera and all feed primarily on plant material, largely fruit, nectar or pollen. They are found only in the Old World tropics. Megachiroptera includes one family (Pteropodidae) and about 166 species. The remaining 16 families (~ 759 species) belong to Microchiroptera. The Microchiroptera are more highly specialized than the Megachiroptera. They are highly varied in appearance, and occur worldwide. They majority of species are largely insectivores, which they locate by emitting ultrasound pulses and listening to the returning echoes (echolocation). Their teeth, like other insectivores, are very sharp to bite through the exoskeleton of insects or the skin of fruit. A small number of bats are carnivorous, hunting such small vertebrates as fish, frogs, mice and birds. -

Bat Wing Sensors Support Flight Control
Bat wing sensors support flight control Susanne Sterbing-D’Angeloa,1, Mohit Chadhab,c, Chen Chiuc, Ben Falkc, Wei Xianc, Janna Barceloc, John M. Zookd, and Cynthia F. Mossa,b,c bProgram in Neuroscience and Cognitive Science, cDepartment of Psychology, and aInstitute for Systems Research, University of Maryland, College Park, MD 20742; and dDepartment of Biological Sciences, Ohio University, Athens, OH 45701 Edited* by Jon H. Kaas, Vanderbilt University, Nashville, TN, and approved May 25, 2011 (received for review January 3, 2011) Bats are the only mammals capable of powered flight, and they hair is long (up to several millimeters), relatively thick (6–18 perform impressive aerial maneuvers like tight turns, hovering, μm), and found close to the ventral forearm, around the leg, and and perching upside down. The bat wing contains five digits, and on the tail membrane (IFM), resembling pelage hair. On the its specialized membrane is covered with stiff, microscopically other membranous parts of the wing, a second type of hair was small, domed hairs. We provide here unique empirical evidence found, which is invisible to the naked eye. Fig. 1 shows an ex- that the tactile receptors associated with these hairs are involved ample of these hairs collected from E.f. This type of hair is very in sensorimotor flight control by providing aerodynamic feedback. short (100–600 μm), with the shortest ones found along the We found that neurons in bat primary somatosensory cortex re- trailing edge of the wing. They are so thin that only one follicle spond with directional sensitivity to stimulation of the wing hairs cell builds each segment of the hair, resulting in a coronal scale with low-speed airflow.