Measuring Cochlear Duct Length – a Historical Analysis of Methods and Results Robert W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
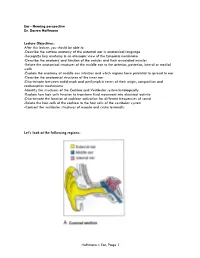
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -

Structure and Function of Ear
STRUCTURE AND FUNCTION OF EAR sns college of pharmacy and allied health 1 sciences EAR The human ear serve as an astounding transducer, converting sound energy mechanical energy nerve impulse which is transmitted to the brain Ear consists of three basic parts Outer ear-collect and channel sound to middle ear Middle ear-transform the energy of soundwave into internal vibration to the bones of middle ear and then into compressional wave in the inner ear Inner ear-compressional wave into nerve impulse which can be transmiited to brain sns college of pharmacy and allied health 2 sciences OUTER EAR: The outer ear consist of Pinna and External auditory meatus Auricle (pinna) : collects and directs sound waves to move the tympanic membrane External auditory canal (meatus) : leads sound waves from Auricle to tympanic membrane . Function : collecting and channeling sound waves into the ear canal . sns college of pharmacy and allied health 3 sciences MIDDLE EAR: Tympanic membrane: Elastic structure which vibrates with sound waves . connected with Auditory ossicles; 3 small bones – malleus , Incus, and stapes which conduct vibrations to oval window of inner ear. Tympanic cavity : Air spaces within temporal bone containing middle ear structures . Eustachian tube : communicates middle ear with pharynx . Muscle tensor tympani stapedius: protect inner ear from loud sounds . Function: conduct and amplifies vibrations through the action of 3 bones. sns college of pharmacy and allied health 4 sciences INNER EAR: It is also called as labyrinth because -

Plan of Lecture Sound Filters External and Middle Ear Cochlear Structure
Plan of lecture Sound Filters External and middle ear Cochlear structure and function 1 2 Analysis of sound by frequency, intensity and timing 3 What does it mean to analyze the frequency components of a sound? A ‘spectrogram’ such as that shown here is the usual display of frequency components as a function of time – here during the production of a sentence “I can see you”. We will see a real-time spectrograph in operation ourselves. 4 ‘audiogram’ of human hearing, with landmarks 5 The frequency composition of speech sounds is shaped by muscular control of the airway. 6 The RC time constant imposes a low frequency limit on the rate at which voltage changes across the cell membrane (or any other system) 7 Current flows across a capacitor in proportion to the rate of change of voltage, Ic = CdV/dt. At steady-state no current flows, so no voltage change is measured. 8 Linked together, low and hi pass result in ‘band pass’. Each cochlear nerve fiber (afferent neuron) behaves as a bandpass filter. The ‘quality’ – Q’ of the filter refers to its narrowness, how cleanly does it segregate its center frequency (resonant frequency) from surrounding frequencies. Center frequency divided by bandpass width (at 3 dB (50% down) or 10 dB below the peak. 9 A cell membrane with voltage-gated potassium channels can exhibit resonance, or the behavior of a bandpass filter. The RC time constant serves as the low pass component and the delayed gating of potassium channels reduces the voltage change from some initial value, so serving as the high pass component. -

Anatomic Moment
Anatomic Moment The Endolymphatic Duct and Sac William W. M. Lo, David L. Daniels, Donald W. Chakeres, Fred H. Linthicum, Jr, John L. Ulmer, Leighton P. Mark, and Joel D. Swartz The endolymphatic duct (ED) and the en- lies in a groove on the posteromedial surface of dolymphatic sac (ES) are the nonsensory com- the vestibule (14), while its major portion is ponents of the endolymph-filled, closed, mem- contained within the short, slightly upwardly branous labyrinth. The ED leads from the arched, horizontal segment of the VA (6, 15). utricular and saccular ducts within the vestibule After entering the VA, the sinus tapers to its through the vestibular aqueduct (VA) to the ES, intermediate segment within the horizontal seg- which extends through the distal VA out the ment of the VA, and then narrows at its isthmus external aperture of the aqueduct (Fig 1) to within the isthmus of the VA (13). The mean terminate in the epidural space of the posterior diameters of the ED, 0.16 3 0.41 mm at the cranial fossa. Thus, the ED-ES system consists internal aperture of the VA and 0.09 3 0.20 mm of components both inside and outside the otic at the isthmus, are below the resolution of capsule connected by a narrow passageway present MR imagers (Fig 6A). The correspond- through the capsule (1). In nomenclature, the ing measurements of the VA, 0.32 3 0.72 and osseous VA should be clearly distinguished 0.18 3 0.31 mm, also challenge the resolution from the membranous ED and ES, which it of current CT scanners. -

Evaluation of Human Ear Anatomy and Functionality by Axiomatic Design
biomimetics Article Evaluation of Human Ear Anatomy and Functionality by Axiomatic Design Pratap Sriram Sundar 1, Chandan Chowdhury 2 and Sagar Kamarthi 3,* 1 Indian School of Business, Mohali 160062, India; [email protected] 2 Indian School of Business, Gachibowli, Hyderabad 500111, India; [email protected] 3 Department of Mechanical and Industrial Engineering, Northeastern University, Boston, MA 02115, USA * Correspondence: [email protected]; Tel.: +1-617-373-3070 Abstract: The design of the human ear is one of nature’s engineering marvels. This paper examines the merit of ear design using axiomatic design principles. The ear is the organ of both hearing and balance. A sensitive ear can hear frequencies ranging from 20 Hz to 20,000 Hz. The vestibular apparatus of the inner ear is responsible for the static and dynamic equilibrium of the human body. The ear is divided into the outer ear, middle ear, and inner ear, which play their respective functional roles in transforming sound energy into nerve impulses interpreted in the brain. The human ear has many modules, such as the pinna, auditory canal, eardrum, ossicles, eustachian tube, cochlea, semicircular canals, cochlear nerve, and vestibular nerve. Each of these modules has several subparts. This paper tabulates and maps the functional requirements (FRs) of these modules onto design parameters (DPs) that nature has already chosen. The “independence axiom” of the axiomatic design methodology is applied to analyze couplings and to evaluate if human ear design is a good design (i.e., uncoupled design) or a bad design (i.e., coupled design). The analysis revealed that the human ear is a perfect design because it is an uncoupled structure. -

The Special Senses the Ear External Ear Middle
1/24/2016 The Ear • The organ of hearing and equilibrium – Cranial nerve VIII - Vestibulocochlear – Regions The Special Senses • External ear • Middle ear Hearing and • Internal ear (labyrinth) Equilibrium External Ear Middle Internal ear • Two parts External ear (labyrinth) ear – Pinna or auricle (external structures) – External auditory meatus (car canal) Auricle • Site of cerumen (earwax) production (pinna) – Waterproofing, protection • Separated from the middle ear by the tympanic membrane Helix (eardrum) – Vibrates in response to sound waves Lobule External acoustic Tympanic Pharyngotympanic meatus membrane (auditory) tube (a) The three regions of the ear Figure 15.25a Middle Ear Epitympanic Middle Ear Superior Malleus Incus recess Lateral • Tympanic cavity Anterior – Air-filled chamber – Openings View • Tympanic membrane – covers opening to outer ear • Round and oval windows – openings to inner ear • Epitympanic recess – dead-end cavity into temporal bone of unknown function • Auditory tube – AKA Eustachian tube or pharyngotympanic tube Pharyngotym- panic tube Tensor Tympanic Stapes Stapedius tympani membrane muscle muscle (medial view) Figure 15.26 1 1/24/2016 Middle Ear Middle Ear • Auditory tube (Eustachian tube) • Otitis Media – Connects the middle ear to the nasopharynx • Equalizes pressure – Opens during swallowing and yawning Middle Ear Middle Ear • Contains auditory ossicles (bones) • Sound waves cause tympanic membrane to vibrate – Malleus • Ossicles help transmit vibrations into the inner ear – Incus – Reduce the area -

The Special Senses
HOMEWORK DUE IN LAB 5 HW page 9: Matching Eye Disorders PreLab 5 THE SPECIAL SENSES Hearing and Equilibrium THE EAR The organ of hearing and equilibrium . Cranial nerve VIII - Vestibulocochlear . Regions . External ear . Middle ear . Internal ear (labyrinth) Middle Internal ear External ear (labyrinth) ear Auricle (pinna) Helix Lobule External acoustic Tympanic Pharyngotympanic meatus membrane (auditory) tube (a) The three regions of the ear Figure 15.25a Middle Ear Epitympanic Superior Malleus Incus recess Lateral Anterior View Pharyngotym- panic tube Tensor Tympanic Stapes Stapedius tympani membrane muscle muscle (medial view) Copyright © 2010 Pearson Education, Inc. Figure 15.26 MIDDLE EAR Auditory tube . Connects the middle ear to the nasopharynx . Equalizes pressure . Opens during swallowing and yawning . Otitis media INNER EAR Contains functional organs for hearing & equilibrium . Bony labyrinth . Membranous labyrinth Superior vestibular ganglion Inferior vestibular ganglion Temporal bone Semicircular ducts in Facial nerve semicircular canals Vestibular nerve Anterior Posterior Lateral Cochlear Cristae ampullares nerve in the membranous Maculae ampullae Spiral organ Utricle in (of Corti) vestibule Cochlear duct Saccule in in cochlea vestibule Stapes in Round oval window window Figure 15.27 INNER EAR - BONY LABYRINTH Three distinct regions . Vestibule . Gravity . Head position . Linear acceleration and deceleration . Semicircular canals . Angular acceleration and deceleration . Cochlea . Vibration Superior vestibular ganglion Inferior vestibular ganglion Temporal bone Semicircular ducts in Facial nerve semicircular canals Vestibular nerve Anterior Posterior Lateral Cochlear Cristae ampullares nerve in the membranous Maculae ampullae Spiral organ Utricle in (of Corti) vestibule Cochlear duct Saccule in in cochlea vestibule Stapes in Round oval window window Figure 15.27 INNER EAR The cochlea . -

2 the Anatomy and Physiology of the Ear and Hearing
2 THE ANATOMY AND PHYSIOLOGY OF THE EAR AND HEARING Peter W.Alberti Professor em. of Otolaryngology Visiting Professor University of Singapore University of Toronto Department of Otolaryngology Toronto 5 Lower Kent Ridge Rd CANADA SINGAPORE 119074 [email protected] 2.1. INTRODUCTION Hearing is one of the major senses and like vision is important for distant warning and communication. It can be used to alert, to communicate pleasure and fear. It is a conscious appreciation of vibration perceived as sound. In order to do this, the appropriate signal must reach the higher parts of the brain. The function of the ear is to convert physical vibration into an encoded nervous impulse. It can be thought of as a biological microphone. Like a microphone the ear is stimulated by vibration: in the microphone the vibration is transduced into an electrical signal, in the ear into a nervous impulse which in turn is then processed by the central auditory pathways of the brain. The mechanism to achieve this is complex. This chapter will deal mainly with the ear, first its structure and then its function, for it is the ear that is mainly at risk from hazardous sounds. The ears are paired organs, one on each side of the head with the sense organ itself, which is technically known as the cochlea, deeply buried within the temporal bones. Part of the ear is concerned with conducting sound to the cochlea, the cochlea is concerned with transducing vibration. The transduction is performed by delicate hair cells which, when stimulated, initiate a nervous impulse. -
Hearing & Equilibrium
Chapter 15 Hearing & Equilibrium ANATOMY OF THE OUTER EAR EAR PINNA is the outer ear…it is thin skin covering elastic cartilage. It directs incoming sound waves to… the EXTERNAL AUDITORY CANAL, which is skin-lined canal containing hair and sebaceous glands. The glands are actually the CERUMINOUS GLANDS, which secrete cerumen. Its purpose is to trap foreign particles. Next, the sound waves go to the… TEMPANIC MEMBRANE (ear drum). It is a think, flattened conical CT membrane. It is covered by skin externally and mucosa internally. ANATOMY OF THE MIDDLE EAR The MIDDLE EAR is air-filled space inside the temporal bone. It is lined by mucosa that is continuous with the pharynx anteromedially via the auditory tube, which is collapsed most of the time. The auditory tube allows equalization of atmospheric pressure so the tempanic membrane can move freely (when the ear has a lot of pressure, the TP is taught and doesn’t vibrate as much. This is why I’m always pretty deaf when I get off a plane.) BONES OF MIDDLE EAR The bones of the middle ear are called OSSICLES. They transmit and amplify tympanic membrane vibrations to the inner ear. o Malleus (hammer) o Incas (anvil) o Stapes (stirrup) OVAL WINDOW is a foramen in medial wall of the middle ear. It is covered by the footplate of the stapes. ROUND WINDOW is inferior to the oval window. It has a diaphragm, meaning it is covered by secondary tympanic membrane. SKELETAL MUSCLES OF THE MIDDLE EAR The muscles of the middle ear reflexively contract to dampen loud sounds. -

Cochlear Anatomy, Function and Pathology I
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University [email protected] Aims and objectives of these lectures • Introduction to gross anatomy of the cochlea • Focus (1) on the sensory epithelium: – Hair cells and the organ of Corti – The mechanism of mechanoelectrical transduction Aims and objectives of these lectures • Focus (2) on the biophysics of the cochlea, the dual roles of hair cells and their innervation: – Cochlear frequency selectivity – The cochlear amplifier – Neurotransmission and innervation of the hair cells – Spiral ganglion and the structure of the auditory nerve Aims and objectives of these lectures • Focus (3) on the cochlear lateral wall and Reissner’s membrane: – The spiral ligament – The stria vascularis – The endolymphatic potential and potassium recycling – Reissner’s membrane Aims and objectives of these lectures • Focus (4) on cochlear pathology: – Presbyacusis – Ototoxicity – Noise trauma – Genetic hearing loss – Molecular mechanisms of cell loss – Regeneration and repair Inner ear From Bear, Connors and Paradiso, Neuroscience: exploring the brain (Lippincott Williams and Wilkins) Cochlea • The main functions of the cochlea are to analyse and convert the vibrations caused by sound into a pattern of electrical signals that can be conveyed along the auditory nerve fibres to the brain • This process involves three main steps: – sensory transduction – processing of the signal – neurotransmission The bony and membraneous labyrinths From Furness and Hackney, Scott-Brown’s Otorhinolaryngology: -

The Anatomy and Anatomical Variations of the Round Window Prechamber and Their Implications on Cochlear Implantation: an Anatomical, Imaging, and Surgical Study
Published online: 2019-12-13 THIEME 288 Original Research The Anatomy and Anatomical Variations of the Round Window Prechamber and Their Implications on Cochlear Implantation: An Anatomical, Imaging, and Surgical Study Ahmed Mohamed Mehanna1 Moustafa Mohamed Abdelnaby1 Mohamed Eid2 1 Department of Otolaryngology, Alexandria University, Midan al Address for correspondence Dr. Ahmed Mohamed Mehanna, Khartoum, Alexandria, Egypt Department of Otolaryngology, Alexandria University, Midan al 2 Department of Radiodiagnosis, Faculty of Medicine of Alexandria Khartoum, Alexandria, Egypt 11511 (e-mail: [email protected]). University, Midan al Khartoum, Alexandria, Egypt Int Arch Otorhinolaryngol 2020;24(3):e288–e298. Abstract Introduction Over the last decades, there has been a tremendous increase in the number of cochlear implant recipients and, consequently, there is a recent increase of interest in the proper understanding of the anatomy of the round window (RW), which is the most important anatomical land mark during cochlear implant surgery. Objectives The present study was undertaken to assess the detailed surgical and radiological anatomy of the RW prechamber; its shape, directions, measurements, common anatomic variations, and its relationships with different surrounding struc- tures as related to cochlear implantation. Methods A total of 20 cadaveric specimens of human temporal bone were micro- scopically dissected for the anatomical assessment of the measurements of the RW and its relation to surrounding structures in the tympanum. A total of 20 patients were subjected to cochlear implantation, and a radiological and surgical assessment of the anatomy of their RW prechambers was performed. Results The distances between the RW and the facial canal (FC), the jugular fossa (JF), the carotid canal (CC), and the oval window (OW) were measured.