Implications for Cochlear Duct Length and Frequency Mapping Luke Helpard1*, Hao Li2,3, Helge Rask-Andersen2,3, Hanif M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sound and the Ear Chapter 2
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter© Jones & Bartlett 2 Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Sound and the Ear © Jones Karen &J. Kushla,Bartlett ScD, Learning, CCC-A, FAAA LLC © Jones & Bartlett Learning, LLC Lecturer NOT School FOR of SALE Communication OR DISTRIBUTION Disorders and Deafness NOT FOR SALE OR DISTRIBUTION Kean University © Jones & Bartlett Key Learning, Terms LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR Acceleration DISTRIBUTION Incus NOT FOR SALE OR Saccule DISTRIBUTION Acoustics Inertia Scala media Auditory labyrinth Inner hair cells Scala tympani Basilar membrane Linear scale Scala vestibuli Bel Logarithmic scale Semicircular canals Boyle’s law Malleus Sensorineural hearing loss Broca’s area © Jones & Bartlett Mass Learning, LLC Simple harmonic© Jones motion (SHM) & Bartlett Learning, LLC Brownian motion Membranous labyrinth Sound Cochlea NOT FOR SALE OR Mixed DISTRIBUTION hearing loss Stapedius muscleNOT FOR SALE OR DISTRIBUTION Compression Organ of Corti Stapes Condensation Osseous labyrinth Tectorial membrane Conductive hearing loss Ossicular chain Tensor tympani muscle Decibel (dB) Ossicles Tonotopic organization © Jones Decibel & hearing Bartlett level (dB Learning, HL) LLC Outer ear © Jones Transducer & Bartlett Learning, LLC Decibel sensation level (dB SL) Outer hair cells Traveling wave theory NOT Decibel FOR sound SALE pressure OR level DISTRIBUTION -

The Standing Acoustic Wave Principle Within the Frequency Analysis Of
inee Eng ring al & ic d M e e d Misun, J Biomed Eng Med Devic 2016, 1:3 m i o c i a B l D f o e v DOI: 10.4172/2475-7586.1000116 l i a c n e r s u o Journal of Biomedical Engineering and Medical Devices J ISSN: 2475-7586 Review Article Open Access The Standing Acoustic Wave Principle within the Frequency Analysis of Acoustic Signals in the Cochlea Vojtech Misun* Department of Solid Mechanics, Mechatronics and Biomechanics, Brno University of Technology, Brno, Czech Republic Abstract The organ of hearing is responsible for the correct frequency analysis of auditory perceptions coming from the outer environment. The article deals with the principles of the analysis of auditory perceptions in the cochlea only, i.e., from the overall signal leaving the oval window to its decomposition realized by the basilar membrane. The paper presents two different methods with the function of the cochlea considered as a frequency analyzer of perceived acoustic signals. First, there is an analysis of the principle that cochlear function involves acoustic waves travelling along the basilar membrane; this concept is one that prevails in the contemporary specialist literature. Then, a new principle with the working name “the principle of standing acoustic waves in the common cavity of the scala vestibuli and scala tympani” is presented and defined in depth. According to this principle, individual structural modes of the basilar membrane are excited by continuous standing waves of acoustic pressure in the scale tympani. Keywords: Cochlea function; Acoustic signals; Frequency analysis; The following is a description of the theories in question: Travelling wave principle; Standing wave principle 1. -

Low-Frequency Noise: a Biophysical Phenomenon M
PSC REF#:288480 Public Service Commission of Wisconsin RECEIVED: 07/08/16, 8:46:12 AM Congres Geluid, Trillingen, Luchtkwaliteit en Gebied & Gebouw 2012 Low-frequency noise: a biophysical phenomenon M. Oud (medical physicist / consultant)* * [email protected], http://nl.linkedin.com/in/mireilleoud, the Netherlands Abstract Complaints on low-frequency noise were till recently fairly unexplained, but audiological research shed light on the mechanisms that enable perception of frequencies below the threshold of average normal hearing. It was shown that exposure to low-frequency sound may alter the inner ear. This results in an increase of sensitivity to low-frequency sounds, and as a result, previously imperceptible sounds becomes audible to the exposed person. Interactions between inner-ear responses to low and higher frequencies furthermore account for perception of low-frequency sound, as well as the property of the hearing system to perceive so-called difference tones. Introduction A growing minority of people experiences an increased sensitivity for low-frequency sound. Not surprisingly, they complain about noise, even about loud noise in some cases. Their complaints about the presence of hum, buzz, and rumble are often not recognized as a nuisance, since the majority of people does not perceive the very low frequencies. Low-frequency noise (LFN) may have serious health effects like vertigo, disturbed sleep, stress, hypertension, and heart rhythm disorders [1]. The number of sufferers is growing, and this has two possible causes. The sources of low- frequency sounds increased in volume and dimension over the past decades, and auditory sensitisation takes years to develop. Nowadays, the main source of low-frequency noise is the public infrastructure: wind turbines, gas transmission grid, industrial plants, road and railway traffic, sewerage, and so on. -
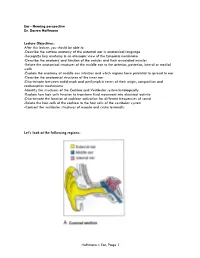
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

CONGENITAL MALFORMATIONS of the INNER EAR Malformaciones Congénitas Del Oído Interno
topic review CONGENITAL MALFORMATIONS OF THE INNER EAR Malformaciones congénitas del oído interno. Revisión de tema Laura Vanessa Ramírez Pedroza1 Hernán Darío Cano Riaño2 Federico Guillermo Lubinus Badillo2 Summary Key words (MeSH) There are a great variety of congenital malformations that can affect the inner ear, Ear with a diversity of physiopathologies, involved altered structures and age of symptom Ear, inner onset. Therefore, it is important to know and identify these alterations opportunely Hearing loss Vestibule, labyrinth to lower the risks of all the complications, being of great importance, among others, Cochlea the alterations in language development and social interactions. Magnetic resonance imaging Resumen Existe una gran variedad de malformaciones congénitas que pueden afectar al Palabras clave (DeCS) oído interno, con distintas fisiopatologías, diferentes estructuras alteradas y edad Oído de aparición de los síntomas. Por lo anterior, es necesario conocer e identificar Oído interno dichas alteraciones, con el fin de actuar oportunamente y reducir el riesgo de las Pérdida auditiva Vestíbulo del laberinto complicaciones, entre otras —de gran importancia— las alteraciones en el área del Cóclea lenguaje y en el ámbito social. Imagen por resonancia magnética 1. Epidemiology • Hyperbilirubinemia Ear malformations occur in 1 in 10,000 or 20,000 • Respiratory distress from meconium aspiration cases (1). One in every 1,000 children has some degree • Craniofacial alterations (3) of sensorineural hearing impairment, with an average • Mechanical ventilation for more than five days age at diagnosis of 4.9 years. The prevalence of hearing • TORCH Syndrome (4) impairment in newborns with risk factors has been determined to be 9.52% (2). -

Measuring Cochlear Duct Length – a Historical Analysis of Methods and Results Robert W
Koch et al. Journal of Otolaryngology - Head and Neck Surgery (2017) 46:19 DOI 10.1186/s40463-017-0194-2 REVIEW Open Access Measuring Cochlear Duct Length – a historical analysis of methods and results Robert W. Koch1*, Hanif M. Ladak1,2,3,4†, Mai Elfarnawany2† and Sumit K. Agrawal1,2,4,5† Abstract Background: Cochlear Duct Length (CDL) has been an important measure for the development and advancement of cochlear implants. Emerging literature has shown CDL can be used in preoperative settings to select the proper sized electrode and develop customized frequency maps. In order to improve post-operative outcomes, and develop new electrode technologies, methods of measuring CDL must be validated to allow usage in the clinic. Purpose: The purpose of this review is to assess the various techniques used to calculate CDL and provide the reader with enough information to make an informed decision on how to conduct future studies measuring the CDL. Results: The methods to measure CDL, the modality used to capture images, and the location of the measurement have all changed as technology evolved. With recent popularity and advancement in computed tomography (CT) imaging in place of histologic sections, measurements of CDL have been focused at the lateral wall (LW) instead of the organ of Corti (OC), due to the inability of CT to view intracochlear structures. After analyzing results from methods such as directly measuring CDL from histology, indirectly reconstructing the shape of the cochlea, and determining CDL based on spiral coefficients, it was determined the three dimensional (3D) reconstruction method is the most reliable method to measure CDL. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -

Structure and Function of Ear
STRUCTURE AND FUNCTION OF EAR sns college of pharmacy and allied health 1 sciences EAR The human ear serve as an astounding transducer, converting sound energy mechanical energy nerve impulse which is transmitted to the brain Ear consists of three basic parts Outer ear-collect and channel sound to middle ear Middle ear-transform the energy of soundwave into internal vibration to the bones of middle ear and then into compressional wave in the inner ear Inner ear-compressional wave into nerve impulse which can be transmiited to brain sns college of pharmacy and allied health 2 sciences OUTER EAR: The outer ear consist of Pinna and External auditory meatus Auricle (pinna) : collects and directs sound waves to move the tympanic membrane External auditory canal (meatus) : leads sound waves from Auricle to tympanic membrane . Function : collecting and channeling sound waves into the ear canal . sns college of pharmacy and allied health 3 sciences MIDDLE EAR: Tympanic membrane: Elastic structure which vibrates with sound waves . connected with Auditory ossicles; 3 small bones – malleus , Incus, and stapes which conduct vibrations to oval window of inner ear. Tympanic cavity : Air spaces within temporal bone containing middle ear structures . Eustachian tube : communicates middle ear with pharynx . Muscle tensor tympani stapedius: protect inner ear from loud sounds . Function: conduct and amplifies vibrations through the action of 3 bones. sns college of pharmacy and allied health 4 sciences INNER EAR: It is also called as labyrinth because -

Microstructures of the Osseous Spiral Laminae in the Bat Cochlea: a Scanning Electron Microscopic Study
Arch. Histol. Cytol., Vol. 55, No. 3 (1992) p. 315-319 Microstructures of the Osseous Spiral Laminae in the Bat Cochlea: A Scanning Electron Microscopic Study Babi r KUcUK2 and Kazuhiro ABE1 Department of Anatomy, Hokkaido University School of Medicine, Sapporo, Hokkaido; and Department of Otolaryngology2, Tokai University School of Medicine, Isehara, Kanagawa, Japan Received May 22, 1992 Summary. The architecture and surface structures of dary osseous spiral laminae. High frequency sounds the primary and secondary osseous spiral laminae in the vibrate the membrane in the basal regions of the cochlea of the bat, an animal able to hear high fre- cochlear duct, while relatively lower frequency quency sounds, were examined by scanning electron sounds vibrate the membrane in the apical regions microscopy to understand the micromechanical adapta- (BEKESY, 1960) . Using the mouse cochlea, we have tions of the bony supportive elements in the inner ear to suggested that the regional vibration pattern of the the specific hearing function. The bat used was Myotis frater kaguyae. basilar membrane is closely related to the base-to- The myotis bat cochlea was seen to consist of a hook apex variations in the morphology of the osseous and a spiral portion with one and three-quarter turns spiral laminae (KUcUK and ABE, 1989). It is known and was characterized by: 1) a distinct ridge-like pro- that the bat cochlea is sensitive to sounds in the very jection running spirally along the middle line on the high frequency range. In fact, the frequencies of the vestibular leaf of the primary osseous spiral lamina; 2) sounds that stimulate the basilar membrane in the a wide secondary osseous spiral lamina; and 3) a narrow bat cochlea are higher than those that stimulate the spiral fissure between the primary and secondary osse- basal regions of the basilar membrane in the mouse ous spiral laminae. -

Plan of Lecture Sound Filters External and Middle Ear Cochlear Structure
Plan of lecture Sound Filters External and middle ear Cochlear structure and function 1 2 Analysis of sound by frequency, intensity and timing 3 What does it mean to analyze the frequency components of a sound? A ‘spectrogram’ such as that shown here is the usual display of frequency components as a function of time – here during the production of a sentence “I can see you”. We will see a real-time spectrograph in operation ourselves. 4 ‘audiogram’ of human hearing, with landmarks 5 The frequency composition of speech sounds is shaped by muscular control of the airway. 6 The RC time constant imposes a low frequency limit on the rate at which voltage changes across the cell membrane (or any other system) 7 Current flows across a capacitor in proportion to the rate of change of voltage, Ic = CdV/dt. At steady-state no current flows, so no voltage change is measured. 8 Linked together, low and hi pass result in ‘band pass’. Each cochlear nerve fiber (afferent neuron) behaves as a bandpass filter. The ‘quality’ – Q’ of the filter refers to its narrowness, how cleanly does it segregate its center frequency (resonant frequency) from surrounding frequencies. Center frequency divided by bandpass width (at 3 dB (50% down) or 10 dB below the peak. 9 A cell membrane with voltage-gated potassium channels can exhibit resonance, or the behavior of a bandpass filter. The RC time constant serves as the low pass component and the delayed gating of potassium channels reduces the voltage change from some initial value, so serving as the high pass component.