Anatomic Moment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Direct Sagittal CT in the Evaluation of Temporal Bone Disease
371 Direct Sagittal CT in the Evaluation of Temporal Bone Disease 1 Mahmood F. Mafee The human temporal bone is an extremely complex structure. Direct axial and coronal Arvind Kumar2 CT sections are quite satisfactory for imaging the anatomy of the temporal bone; Christina N. Tahmoressi1 however, many relationships of the normal and pathologic anatomic detail of the Barry C. Levin2 temporal bone are better seen with direct sagittal CT sections. The sagittal projection Charles F. James1 is of interest to surgeons, as it has the advantage of following the plane of surgical approach. This article describes the advantages of using direct sagittal sections for Robert Kriz 1 1 studying various diseases of the temporal bone. The CT sections were obtained with Vlastimil Capek the aid of a new headholder added to our GE CT 9800 scanner. The direct sagittal projection was found to be extremely useful for evaluating diseases involving the vertical segment of the facial nerve canal, vestibular aqueduct, tegmen tympani, sigmoid sinus plate, sinodural angle, carotid canal, jugular fossa, external auditory canal, middle ear cavity, infra- and supra labyrinthine air cells, and temporo mandibular joint. CT has contributed greatly to an understanding of the complex anatomy and spatial relationship of the minute structures of the hearing and balance organs, which are packed into a small pyramid-shaped petrous temporal bone [1 , 2]. In the past 6 years, high-resolution CT scanning has been rapidly replacing standard tomography and has proved to be the diagnostic imaging method of choice for studying the normal and pathologic details of the temporal bone [3-14]. -

Endolymphatic Sac Tumors: an Overview Michael B
Endolymphatic Sac Tumors: An Overview Michael B. Gluth, MD, FACS Associate Professor Director, Comprehensive Ear & Hearing Ctr. Section of Otolaryngology-Head & Neck Surgery VHL Alliance, Chicago, 2015 Disclosure • No relevant financial interests or other relevant relationships to disclose • Will discuss off-label use of cochlear implants for single-sided SNHL VHL | 2 Outline • What is an endolymphatic sac tumor? • What are the symptoms? • What is the work-up and treatment? VHL | 3 Endolymphatic Sac Anatomy VHL | 4 Endolymphatic Sac Function • Part of the “membranous labyrinth” • Filled with fluid called endolymph • Secretes locally acting chemical called “saccin” • Involved with inner ear fluid homeostasis, mechanisms not fully understood – ELS is involved with Meniere’s disease VHL | 5 ELS Tumors • Extremely rare tumor originating from the endolymphatic sac, only recognized as a unique entity since 1989 • Benign (not cancer) • Highly destructive, slowly progressive – Destroy bone of inner ear, around cranial vault/skull base, and around facial nerve – Can grow into nerves, pass through dura (sac around brain) and press on cerebellum • May be asymptomatic until inner ear is partially destroyed • Key: if hearing loss is present: high chance dura is invaded VHL | 6 Symptoms • Most common presenting symptoms: – Hearing loss (85%) – Visible mass in the ear (50%) – Ringing in the ear (48%) – Facial paralysis (44%) – Dizziness/imbalance (44%) – Headache (37%) – Other neurologic (cranial nerve) weakness (25%) • If present in both ears, outlook -

Bachmann.Pdf
Pediatric Vestibular Dysfunction: More Than a Balancing Act Kay Bachmann, PhD 1 2 Introduction Childhood Hearing Loss 1. The learner will be able to identify 3 causes of vestibular • 1.4 in 1,000 newborns have hearing loss (CDC) disorders in children. • 5 in 1,000 children have hearing loss ages 3-17 yrs (CDC) 2. The learner will be able to identify at least 3 common symptoms of vestibular dysfunction in children. 3. The learner will be able to administer a short screening to assess balance function in children. 3 4 1 Childhood Vestibular Loss • Balance disorders may make up .45% of chief complaints per chart review in a Pediatric ENT department. (O’Reilly et al 2010) • Children with hearing impairment are twice as likely to have vestibular loss than healthy children • Studies show vestibular loss in 30-79% of children with HL • There is 10% increase in vestibular loss as a result of trauma from receiving a CI (Jacot et al, 2009) 5 6 Common Disorders with Hearing Loss and Vestibular Abnormalities • Syndromes What Does a Typically Functioning Vestibular System Do? – Usher Syndrome Type 1 – Pendred Syndrome (also non syndromic Enlarged vestibular Aqueduct Syndrome) Two Main Reflexes: – Branchio-oto-renal Syndrome Vestibular Ocular Reflex- Helps hold an object – CHARGE association steady when the head/body are in motion. • Cochlear Malformations • VIII Nerve Defects • Cytomegalovirus (CMV) Vestibular Spinal Reflex- Helps keep our posture • Meningitis • Cochlear implant patients and body steady when it senses movement. 7 8 2 What Does an -

Morphological and Functional Changes in a New Animal Model Of
Laboratory Investigation (2013) 93, 1001–1011 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Morphological and functional changes in a new animal model of Me´nie`re’s disease Naoya Egami1, Akinobu Kakigi1, Takashi Sakamoto1, Taizo Takeda2, Masamitsu Hyodo2 and Tatsuya Yamasoba1 The purpose of this study was to clarify the underlying mechanism of vertiginous attacks in Me´nie`re’s disease (MD) while obtaining insight into water homeostasis in the inner ear using a new animal model. We conducted both histopatho- logical and functional assessment of the vestibular system in the guinea-pig. In the first experiment, all animals were maintained 1 or 4 weeks after electrocauterization of the endolymphatic sac of the left ear and were given either saline or desmopressin (vasopressin type 2 receptor agonist). The temporal bones from both ears were harvested and the extent of endolymphatic hydrops was quantitatively assessed. In the second experiment, either 1 or 4 weeks after surgery, animals were assessed for balance disorders and nystagmus after the administration of saline or desmopressin. In the first experiment, the proportion of endolymphatic space in the cochlea and the saccule was significantly greater in ears that survived for 4 weeks after surgery and were given desmopressin compared with other groups. In the second experiment, all animals that underwent surgery and were given desmopressin showed spontaneous nystagmus and balance disorder, whereas all animals that had surgery but without desmopressin administration were asymptomatic. Our animal model induced severe endolymphatic hydrops in the cochlea and the saccule, and showed episodes of balance disorder along with spontaneous nystagmus. -

Saccule and Utricle
THE SPECIAL SENSES VESTIBULAR FUNCTION DR SYED SHAHID HABIB MBBS DSDM PGDCR FCPS Professor Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe: Functional anatomy of Vestibular apparatus Dynamic and static equilibrium Role of utricle and saccule in linear acceleration Role of semicircular canals in angular motions Vestibular Reflexes Overview of Static Proprioception & Balance position sense (Ia) Dynamic position sense (II) Static Equilibrium Utricle & Saccule Neck Proprioceptors Linear Acceleration Horizontal (Utricle) Visual Information (vesitbulo Ocular) Linear Acceleration Vestibular Apparatus Horizontal (Saccule) Proprioception Chest Wall Equilibrium Angular Acceleration Proprioceptors (SCCs) air pressure against body Predictive Functions (SCCs) Footpads pressure To balance the centre of gravity must be above the support point. Centre of gravity Physiology Of Body Balance Balance & Equilibrium Balance is the ability to maintain the equilibrium of the body • Foot position affects standing balance Equilibrium is the state of a body or physical system at rest or in un accelerated motion in which the resultant of all forces acting on it is zero and the sum of all torques about any axis is zero. There are 2 types of Equilibrium » Static - » Dynamic – Static Equilibrium keep the body in a desired position Static equilibrium –The equilibrium is maintained in a FIXED POSITION, usually while stood on one foot or maintenance of body posture relative to gravity while the body is still. Dynamic Equilibrium to move the body in a controlled way Dynamic equilibrium The equilibrium must be maintained while performing a task which involves MOVEMENT e.g. Walking the beam. -
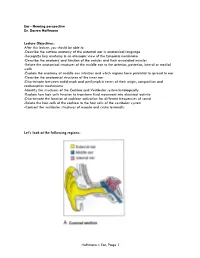
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

CONGENITAL MALFORMATIONS of the INNER EAR Malformaciones Congénitas Del Oído Interno
topic review CONGENITAL MALFORMATIONS OF THE INNER EAR Malformaciones congénitas del oído interno. Revisión de tema Laura Vanessa Ramírez Pedroza1 Hernán Darío Cano Riaño2 Federico Guillermo Lubinus Badillo2 Summary Key words (MeSH) There are a great variety of congenital malformations that can affect the inner ear, Ear with a diversity of physiopathologies, involved altered structures and age of symptom Ear, inner onset. Therefore, it is important to know and identify these alterations opportunely Hearing loss Vestibule, labyrinth to lower the risks of all the complications, being of great importance, among others, Cochlea the alterations in language development and social interactions. Magnetic resonance imaging Resumen Existe una gran variedad de malformaciones congénitas que pueden afectar al Palabras clave (DeCS) oído interno, con distintas fisiopatologías, diferentes estructuras alteradas y edad Oído de aparición de los síntomas. Por lo anterior, es necesario conocer e identificar Oído interno dichas alteraciones, con el fin de actuar oportunamente y reducir el riesgo de las Pérdida auditiva Vestíbulo del laberinto complicaciones, entre otras —de gran importancia— las alteraciones en el área del Cóclea lenguaje y en el ámbito social. Imagen por resonancia magnética 1. Epidemiology • Hyperbilirubinemia Ear malformations occur in 1 in 10,000 or 20,000 • Respiratory distress from meconium aspiration cases (1). One in every 1,000 children has some degree • Craniofacial alterations (3) of sensorineural hearing impairment, with an average • Mechanical ventilation for more than five days age at diagnosis of 4.9 years. The prevalence of hearing • TORCH Syndrome (4) impairment in newborns with risk factors has been determined to be 9.52% (2). -

Genetic Determinants of Non-Syndromic Enlarged Vestibular Aqueduct: a Review
Review Genetic Determinants of Non-Syndromic Enlarged Vestibular Aqueduct: A Review Sebastian Roesch 1 , Gerd Rasp 1, Antonio Sarikas 2 and Silvia Dossena 2,* 1 Department of Otorhinolaryngology, Head and Neck Surgery, Paracelsus Medical University, 5020 Salzburg, Austria; [email protected] (S.R.); [email protected] (G.R.) 2 Institute of Pharmacology and Toxicology, Paracelsus Medical University, 5020 Salzburg, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-(0)662-2420-80564 Abstract: Hearing loss is the most common sensorial deficit in humans and one of the most common birth defects. In developed countries, at least 60% of cases of hearing loss are of genetic origin and may arise from pathogenic sequence alterations in one of more than 300 genes known to be involved in the hearing function. Hearing loss of genetic origin is frequently associated with inner ear malformations; of these, the most commonly detected is the enlarged vestibular aqueduct (EVA). EVA may be associated to other cochleovestibular malformations, such as cochlear incomplete partitions, and can be found in syndromic as well as non-syndromic forms of hearing loss. Genes that have been linked to non-syndromic EVA are SLC26A4, GJB2, FOXI1, KCNJ10, and POU3F4. SLC26A4 and FOXI1 are also involved in determining syndromic forms of hearing loss with EVA, which are Pendred syndrome and distal renal tubular acidosis with deafness, respectively. In Caucasian cohorts, approximately 50% of cases of non-syndromic EVA are linked to SLC26A4 and a large fraction of Citation: Roesch, S.; Rasp, G.; patients remain undiagnosed, thus providing a strong imperative to further explore the etiology of Sarikas, A.; Dossena, S. -

Measuring Cochlear Duct Length – a Historical Analysis of Methods and Results Robert W
Koch et al. Journal of Otolaryngology - Head and Neck Surgery (2017) 46:19 DOI 10.1186/s40463-017-0194-2 REVIEW Open Access Measuring Cochlear Duct Length – a historical analysis of methods and results Robert W. Koch1*, Hanif M. Ladak1,2,3,4†, Mai Elfarnawany2† and Sumit K. Agrawal1,2,4,5† Abstract Background: Cochlear Duct Length (CDL) has been an important measure for the development and advancement of cochlear implants. Emerging literature has shown CDL can be used in preoperative settings to select the proper sized electrode and develop customized frequency maps. In order to improve post-operative outcomes, and develop new electrode technologies, methods of measuring CDL must be validated to allow usage in the clinic. Purpose: The purpose of this review is to assess the various techniques used to calculate CDL and provide the reader with enough information to make an informed decision on how to conduct future studies measuring the CDL. Results: The methods to measure CDL, the modality used to capture images, and the location of the measurement have all changed as technology evolved. With recent popularity and advancement in computed tomography (CT) imaging in place of histologic sections, measurements of CDL have been focused at the lateral wall (LW) instead of the organ of Corti (OC), due to the inability of CT to view intracochlear structures. After analyzing results from methods such as directly measuring CDL from histology, indirectly reconstructing the shape of the cochlea, and determining CDL based on spiral coefficients, it was determined the three dimensional (3D) reconstruction method is the most reliable method to measure CDL. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Endolymphatic Hydrops Meniere's
ENDOLYMPHATIC HYDROPS MENIERE’S DISEASE Introduction: Meniere’s Disease, or endolymphatic hydrops, is a disorder of the inner ear. This condition occurs because of abnormal fluctuations in the inner ear fluid called endolymph. A system of membranes, called the membranous labyrinth, contains a fluid called endolymph. This fluid bathes the inner ear balance and hearing system sensory cells and allows them to function normally. The membranes can become dilated like a balloon when pressure increases. This is called "hydrops". The amount of fluid is normally kept constant by altering the production and absorption of the fluid. Endolymph also contains a specific concentration of sodium, potassium, chloride, and other electrolytes. If the inner ear is damaged by disease, injury, or other causes, the volume and composition of the inner ear fluid can fluctuate with changes in the body’s fluid and electrolyte levels. This fluctuation in inner ear fluid can cause the symptoms of hydrops, including pressure or fullness of the affected ear, tinnitus, hearing loss, and imbalance or dizziness. Treatment of this condition is geared towards stabilizing the body fluid levels so that fluctuations in the endolymph volume can be avoided. Meniere's episodes may occur in clusters in which several attacks may occur within a short period of time. On the other hand, years may pass between episodes. Between the acute attacks, most people are free of symptoms or note mild imbalance, tinnitus, and/or hearing loss. Meniere’s affects roughly 0.2% of the population, about 200 out of 100,000 people (or in other words, 2/1000). -

Organ of Corti Size Is Governed by Yap/Tead-Mediated Progenitor Self-Renewal
Organ of Corti size is governed by Yap/Tead-mediated progenitor self-renewal Ksenia Gnedevaa,b,1, Xizi Wanga,b, Melissa M. McGovernc, Matthew Bartond,2, Litao Taoa,b, Talon Treceka,b, Tanner O. Monroee,f, Juan Llamasa,b, Welly Makmuraa,b, James F. Martinf,g,h, Andrew K. Grovesc,g,i, Mark Warchold, and Neil Segila,b,1 aDepartment of Stem Cell Biology and Regenerative Medicine, Keck Medicine of University of Southern California, Los Angeles, CA 90033; bCaruso Department of Otolaryngology–Head and Neck Surgery, Keck Medicine of University of Southern California, Los Angeles, CA 90033; cDepartment of Neuroscience, Baylor College of Medicine, Houston, TX 77030; dDepartment of Otolaryngology, Washington University in St. Louis, St. Louis, MO 63130; eAdvanced Center for Translational and Genetic Medicine, Lurie Children’s Hospital of Chicago, Chicago, IL 60611; fDepartment of Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, TX 77030; gProgram in Developmental Biology, Baylor College of Medicine, Houston, TX 77030; hCardiomyocyte Renewal Laboratory, Texas Heart Institute, Houston, TX 77030 and iDepartment of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030; Edited by Marianne E. Bronner, California Institute of Technology, Pasadena, CA, and approved April 21, 2020 (received for review January 6, 2020) Precise control of organ growth and patterning is executed However, what initiates this increase in Cdkn1b expression re- through a balanced regulation of progenitor self-renewal and dif- mains unclear. In addition, conditional ablation of Cdkn1b in the ferentiation. In the auditory sensory epithelium—the organ of inner ear is not sufficient to completely relieve the block on Corti—progenitor cells exit the cell cycle in a coordinated wave supporting cell proliferation (9, 10), suggesting the existence of between E12.5 and E14.5 before the initiation of sensory receptor additional repressive mechanisms.