Anatomic Moment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiological Mechanisms and Functional Hearing Consequences of Auditory Neuropathy
doi:10.1093/brain/awv270 BRAIN 2015: 138; 3141–3158 | 3141 REVIEW ARTICLE Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy Gary Rance1 and Arnold Starr2 The effects of inner ear abnormality on audibility have been explored since the early 20th century when sound detection measures were first used to define and quantify ‘hearing loss’. The development in the 1970s of objective measures of cochlear hair cell Downloaded from function (cochlear microphonics, otoacoustic emissions, summating potentials) and auditory nerve/brainstem activity (auditory brainstem responses) have made it possible to distinguish both synaptic and auditory nerve disorders from sensory receptor loss. This distinction is critically important when considering aetiology and management. In this review we address the clinical and pathophysiological features of auditory neuropathy that distinguish site(s) of dysfunction. We describe the diagnostic criteria for: (i) presynaptic disorders affecting inner hair cells and ribbon synapses; (ii) postsynaptic disorders affecting unmyelinated auditory http://brain.oxfordjournals.org/ nerve dendrites; (iii) postsynaptic disorders affecting auditory ganglion cells and their myelinated axons and dendrites; and (iv) central neural pathway disorders affecting the auditory brainstem. We review data and principles to identify treatment options for affected patients and explore their benefits as a function of site of lesion. 1 Department of Audiology and Speech Pathology, The University of Melbourne, -

Perforated Eardrum
Vinod K. Anand, MD, FACS Nose and Sinus Clinic Perforated Eardrum A perforated eardrum is a hole or rupture m the eardrum, a thin membrane which separated the ear canal and the middle ear. The medical term for eardrum is tympanic membrane. The middle ear is connected to the nose by the eustachian tube. A perforated eardrum is often accompanied by decreased hearing and occasional discharge. Paih is usually not persistent. Causes of Eardrum Perforation The causes of perforated eardrum are usually from trauma or infection. A perforated eardrum can occur: if the ear is struck squarely with an open hand with a skull fracture after a sudden explosion if an object (such as a bobby pin, Q-tip, or stick) is pushed too far into the ear canal. as a result of hot slag (from welding) or acid entering the ear canal Middle ear infections may cause pain, hearing loss and spontaneous rupture (tear) of the eardrum resulting in a perforation. In this circumstance, there may be infected or bloody drainage from the ear. In medical terms, this is called otitis media with perforation. On rare occasions a small hole may remain in the eardrum after a previously placed P.E. tube (pressure equalizing) either falls out or is removed by the physician. Most eardrum perforations heal spontaneously within weeks after rupture, although some may take up to several months. During the healing process the ear must be protected from water and trauma. Those eardrum perforations which do not heal on their own may require surgery. Effects on Hearing from Perforated Eardrum Usually, the larger the perforation, the greater the loss of hearing. -

The Standing Acoustic Wave Principle Within the Frequency Analysis Of
inee Eng ring al & ic d M e e d Misun, J Biomed Eng Med Devic 2016, 1:3 m i o c i a B l D f o e v DOI: 10.4172/2475-7586.1000116 l i a c n e r s u o Journal of Biomedical Engineering and Medical Devices J ISSN: 2475-7586 Review Article Open Access The Standing Acoustic Wave Principle within the Frequency Analysis of Acoustic Signals in the Cochlea Vojtech Misun* Department of Solid Mechanics, Mechatronics and Biomechanics, Brno University of Technology, Brno, Czech Republic Abstract The organ of hearing is responsible for the correct frequency analysis of auditory perceptions coming from the outer environment. The article deals with the principles of the analysis of auditory perceptions in the cochlea only, i.e., from the overall signal leaving the oval window to its decomposition realized by the basilar membrane. The paper presents two different methods with the function of the cochlea considered as a frequency analyzer of perceived acoustic signals. First, there is an analysis of the principle that cochlear function involves acoustic waves travelling along the basilar membrane; this concept is one that prevails in the contemporary specialist literature. Then, a new principle with the working name “the principle of standing acoustic waves in the common cavity of the scala vestibuli and scala tympani” is presented and defined in depth. According to this principle, individual structural modes of the basilar membrane are excited by continuous standing waves of acoustic pressure in the scale tympani. Keywords: Cochlea function; Acoustic signals; Frequency analysis; The following is a description of the theories in question: Travelling wave principle; Standing wave principle 1. -

Experimental Studies on the Function of the Stapedius Muscle Inman
EXPERIMENTAL STUDIES ON THE FUNCTION OF THE STAPEDIUS MUSCLE INMAN AKADEMISK AVHANDLING som med vederbörligt tillstånd av Medicinska fakulteten vid Umeå Universitet för vinnande av medicine doktorsgrad offentligen försvaras i Samhällsvetarhuset, sal D, lördagen den 25 maj 1974 kl. 9.15 f.m. av JOHN-ERIK ZAKRISSON med.lic. UMEÅ 1974 UMEÀ UNIVERSITY MEDICAL DISSERTATIONS No. 18 1974 From the Department of Otorhinolaryngology, University of Umeå, Umeå, Sweden and the Division of Physiological Acoustics, Department of Physiology II, Karolinska Institutet, Stockholm, Sweden EXPERIMENTAL STUDIES ON THE FUNCTION OF THE STAPEDIUS MUSCLE IN MAN BY JOHN-ERIK ZAKRISSON UMEÂ 1974 To Karin Eva and Gunilla The present thesis is based on the following papers which will be referred to in the text by the Roman numerals: I. Zakrisson, J.-E., Borg, E. & Blom, S. The acoustic impedance change as a measure of stapedius muscle activity in man. A methodological study with electromyography. Acta Otolaryng, preprint. II. Borg, E. & Zakrisson, J.-E. Stapedius reflex and monaural masking. Acta Otolaryng, preprint. III. Zakrisson, J.-E. The role of the stapedius reflex in poststimulatory audi tory fatigue. Acta Otolaryng, preprint. IV. Borg, E. & Zakrisson, J.-E. The activity of the stapedius muscle in man during vocalization. Acta Otolaryng, accepted for publication. CONTENTS ABBREVIATIONS .......................................... 8 INTRODUCTION.............................................................................................. 9 MATERIAL..................................................................................................... -

SENSORY MOTOR COORDINATION in ROBONAUT Richard Alan Peters
SENSORY MOTOR COORDINATION IN ROBONAUT 5 Richard Alan Peters 11 Vanderbilt University School of Engineering JSC Mail Code: ER4 30 October 2000 Robert 0. Ambrose Robotic Systems Technology Branch Automation, Robotics, & Simulation Division Engineering Directorate Richard Alan Peters II Robert 0. Ambrose SENSORY MOTOR COORDINATION IN ROBONAUT Final Report NASNASEE Summer Faculty Fellowship Program - 2000 Johnson Space Center Prepared By: Richard Alan Peters II, Ph.D. Academic Rank: Associate Professor University and Department: Vanderbilt University Department of Electrical Engineering and Computer Science Nashville, TN 37235 NASNJSC Directorate: Engineering Division: Automation, Robotics, & Simulation Branch: Robotic Systems Technology JSC Colleague: Robert 0. Ambrose Date Submitted: 30 October 2000 Contract Number: NAG 9-867 13-1 ABSTRACT As a participant of the year 2000 NASA Summer Faculty Fellowship Program, I worked with the engineers of the Dexterous Robotics Laboratory at NASA Johnson Space Center on the Robonaut project. The Robonaut is an articulated torso with two dexterous arms, left and right five-fingered hands, and a head with cameras mounted on an articulated neck. This advanced space robot, now dnven only teleoperatively using VR gloves, sensors and helmets, is to be upgraded to a thinking system that can find, in- teract with and assist humans autonomously, allowing the Crew to work with Robonaut as a (junior) member of their team. Thus, the work performed this summer was toward the goal of enabling Robonaut to operate autonomously as an intelligent assistant to as- tronauts. Our underlying hypothesis is that a robot can deveZop intelligence if it learns a set of basic behaviors ([.e., reflexes - actions tightly coupled to sensing) and through experi- ence learns how to sequence these to solve problems or to accomplish higher-level tasks. -

Morphological and Functional Changes in a New Animal Model Of
Laboratory Investigation (2013) 93, 1001–1011 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Morphological and functional changes in a new animal model of Me´nie`re’s disease Naoya Egami1, Akinobu Kakigi1, Takashi Sakamoto1, Taizo Takeda2, Masamitsu Hyodo2 and Tatsuya Yamasoba1 The purpose of this study was to clarify the underlying mechanism of vertiginous attacks in Me´nie`re’s disease (MD) while obtaining insight into water homeostasis in the inner ear using a new animal model. We conducted both histopatho- logical and functional assessment of the vestibular system in the guinea-pig. In the first experiment, all animals were maintained 1 or 4 weeks after electrocauterization of the endolymphatic sac of the left ear and were given either saline or desmopressin (vasopressin type 2 receptor agonist). The temporal bones from both ears were harvested and the extent of endolymphatic hydrops was quantitatively assessed. In the second experiment, either 1 or 4 weeks after surgery, animals were assessed for balance disorders and nystagmus after the administration of saline or desmopressin. In the first experiment, the proportion of endolymphatic space in the cochlea and the saccule was significantly greater in ears that survived for 4 weeks after surgery and were given desmopressin compared with other groups. In the second experiment, all animals that underwent surgery and were given desmopressin showed spontaneous nystagmus and balance disorder, whereas all animals that had surgery but without desmopressin administration were asymptomatic. Our animal model induced severe endolymphatic hydrops in the cochlea and the saccule, and showed episodes of balance disorder along with spontaneous nystagmus. -
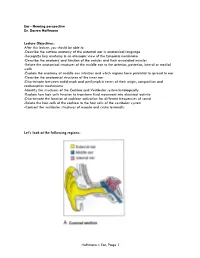
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Vestibular Neuritis and Labyrinthitis
Vestibular Neuritis and DISORDERS Labyrinthitis: Infections of the Inner Ear By Charlotte L. Shupert, PhD with contributions from Bridget Kulick, PT and the Vestibular Disorders Association INFECTIONS Result in damage to inner ear and/or nerve. ARTICLE 079 DID THIS ARTICLE HELP YOU? SUPPORT VEDA @ VESTIBULAR.ORG Vestibular neuritis and labyrinthitis are disorders resulting from an 5018 NE 15th Ave. infection that inflames the inner ear or the nerves connecting the inner Portland, OR 97211 ear to the brain. This inflammation disrupts the transmission of sensory 1-800-837-8428 information from the ear to the brain. Vertigo, dizziness, and difficulties [email protected] with balance, vision, or hearing may result. vestibular.org Infections of the inner ear are usually viral; less commonly, the cause is bacterial. Such inner ear infections are not the same as middle ear infections, which are the type of bacterial infections common in childhood affecting the area around the eardrum. VESTIBULAR.ORG :: 079 / DISORDERS 1 INNER EAR STRUCTURE AND FUNCTION The inner ear consists of a system of fluid-filled DEFINITIONS tubes and sacs called the labyrinth. The labyrinth serves two functions: hearing and balance. Neuritis Inflamation of the nerve. The hearing function involves the cochlea, a snail- shaped tube filled with fluid and sensitive nerve Labyrinthitis Inflamation of the labyrinth. endings that transmit sound signals to the brain. Bacterial infection where The balance function involves the vestibular bacteria infect the middle organs. Fluid and hair cells in the three loop-shaped ear or the bone surrounding semicircular canals and the sac-shaped utricle and Serous the inner ear produce toxins saccule provide the brain with information about Labyrinthitis that invade the inner ear via head movement. -

Vestibular Neuritis, Labyrinthitis, and a Few Comments Regarding Sudden Sensorineural Hearing Loss Marcello Cherchi
Vestibular neuritis, labyrinthitis, and a few comments regarding sudden sensorineural hearing loss Marcello Cherchi §1: What are these diseases, how are they related, and what is their cause? §1.1: What is vestibular neuritis? Vestibular neuritis, also called vestibular neuronitis, was originally described by Margaret Ruth Dix and Charles Skinner Hallpike in 1952 (Dix and Hallpike 1952). It is currently suspected to be an inflammatory-mediated insult (damage) to the balance-related nerve (vestibular nerve) between the ear and the brain that manifests with abrupt-onset, severe dizziness that lasts days to weeks, and occasionally recurs. Although vestibular neuritis is usually regarded as a process affecting the vestibular nerve itself, damage restricted to the vestibule (balance components of the inner ear) would manifest clinically in a similar way, and might be termed “vestibulitis,” although that term is seldom applied (Izraeli, Rachmel et al. 1989). Thus, distinguishing between “vestibular neuritis” (inflammation of the vestibular nerve) and “vestibulitis” (inflammation of the balance-related components of the inner ear) would be difficult. §1.2: What is labyrinthitis? Labyrinthitis is currently suspected to be due to an inflammatory-mediated insult (damage) to both the “hearing component” (the cochlea) and the “balance component” (the semicircular canals and otolith organs) of the inner ear (labyrinth) itself. Labyrinthitis is sometimes also termed “vertigo with sudden hearing loss” (Pogson, Taylor et al. 2016, Kim, Choi et al. 2018) – and we will discuss sudden hearing loss further in a moment. Labyrinthitis usually manifests with severe dizziness (similar to vestibular neuritis) accompanied by ear symptoms on one side (typically hearing loss and tinnitus). -

Inner Ear Infection (Otitis Interna) in Dogs
Hurricane Harvey Client Education Kit Inner Ear Infection (Otitis Interna) in Dogs My dog has just been diagnosed with an inner ear infection. What is this? Inflammation of the inner ear is called otitis interna, and it is most often caused by an infection. The infectious agent is most commonly bacterial, although yeast and fungus can also be implicated in an inner ear infection. If your dog has ear mites in the external ear canal, this can ultimately cause a problem in the inner ear and pose a greater risk for a bacterial infection. Similarly, inner ear infections may develop if disease exists in one ear canal or when a benign polyp is growing from the middle ear. A foreign object, such as grass seed, may also set the stage for bacterial infection in the inner ear. Are some dogs more susceptible to inner ear infection? Dogs with long, heavy ears seem to be predisposed to chronic ear infections that ultimately lead to otitis interna. Spaniel breeds, such as the Cocker spaniel, and hound breeds, such as the bloodhound and basset hound, are the most commonly affected breeds. Regardless of breed, any dog with a chronic ear infection that is difficult to control may develop otitis interna if the eardrum (tympanic membrane) is damaged as it allows bacteria to migrate down into the inner ear. "Dogs with long, heavy ears seem to bepredisposed to chronic ear infections that ultimately lead to otitis interna." Excessively vigorous cleaning of an infected external ear canal can sometimes cause otitis interna. Some ear cleansers are irritating to the middle and inner ear and can cause signs of otitis interna if the eardrum is damaged and allows some of the solution to penetrate too deeply. -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

Vascular Supply of the Human Spiral Ganglion: Novel Three
www.nature.com/scientificreports Corrected: Publisher Correction OPEN Vascular Supply of the Human Spiral Ganglion: Novel Three- Dimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology Xueshuang Mei1,2*, Rudolf Glueckert3, Annelies Schrott-Fischer3, Hao Li1, Hanif M. Ladak4,6, Sumit K. Agrawal5,6 & Helge Rask-Andersen1,6* Human spiral ganglion (HSG) cell bodies located in the bony cochlea depend on a rich vascular supply to maintain excitability. These neurons are targeted by cochlear implantation (CI) to treat deafness, and their viability is critical to ensure successful clinical outcomes. The blood supply of the HSG is difcult to study due to its helical structure and encasement in hard bone. The objective of this study was to present the frst three-dimensional (3D) reconstruction and analysis of the HSG blood supply using synchrotron radiation phase-contrast imaging (SR-PCI) in combination with histological analyses of archival human cochlear sections. Twenty-six human temporal bones underwent SR-PCI. Data were processed using volume-rendering software, and a representative three-dimensional (3D) model was created to allow visualization of the vascular anatomy. Histologic analysis was used to verify the segmentations. Results revealed that the HSG is supplied by radial vascular twigs which are separate from the rest of the inner ear and encased in bone. Unlike with most organs, the arteries and veins in the human cochlea do not follow the same conduits. There is a dual venous outfow and a modiolar arterial supply. This organization may explain why the HSG may endure even in cases of advanced cochlear pathology. Human inner ear function relies on microcirculation derived from vessels in the internal auditory canal (IAC).