Cochlear Partition Anatomy and Motion in Humans Differ from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vascular Supply of the Human Spiral Ganglion: Novel Three
www.nature.com/scientificreports Corrected: Publisher Correction OPEN Vascular Supply of the Human Spiral Ganglion: Novel Three- Dimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology Xueshuang Mei1,2*, Rudolf Glueckert3, Annelies Schrott-Fischer3, Hao Li1, Hanif M. Ladak4,6, Sumit K. Agrawal5,6 & Helge Rask-Andersen1,6* Human spiral ganglion (HSG) cell bodies located in the bony cochlea depend on a rich vascular supply to maintain excitability. These neurons are targeted by cochlear implantation (CI) to treat deafness, and their viability is critical to ensure successful clinical outcomes. The blood supply of the HSG is difcult to study due to its helical structure and encasement in hard bone. The objective of this study was to present the frst three-dimensional (3D) reconstruction and analysis of the HSG blood supply using synchrotron radiation phase-contrast imaging (SR-PCI) in combination with histological analyses of archival human cochlear sections. Twenty-six human temporal bones underwent SR-PCI. Data were processed using volume-rendering software, and a representative three-dimensional (3D) model was created to allow visualization of the vascular anatomy. Histologic analysis was used to verify the segmentations. Results revealed that the HSG is supplied by radial vascular twigs which are separate from the rest of the inner ear and encased in bone. Unlike with most organs, the arteries and veins in the human cochlea do not follow the same conduits. There is a dual venous outfow and a modiolar arterial supply. This organization may explain why the HSG may endure even in cases of advanced cochlear pathology. Human inner ear function relies on microcirculation derived from vessels in the internal auditory canal (IAC). -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

The Membranous Labyrinth in Vivo from High-Resolution Temporal CT Data
bioRxiv preprint doi: https://doi.org/10.1101/318030; this version posted May 9, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. The Membranous Labyrinth in vivo from high-resolution Temporal CT data Hisaya Tanioka¹* ¹Department of Radiology, Tanioka Clinic *Corresponding author Hisaya Tanioka, MD, PhD Tanioka Clinic Tanioka Bldg. 3F, 6-24-2 Honkomagome Bunkyo-ku, Tokyo 113-0021 Japan Tel & Fax: 81-3-3945-5199 E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/318030; this version posted May 9, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. The Membranous Labyrinth in vivo from high-resolution Temporal CT data bioRxiv preprint doi: https://doi.org/10.1101/318030; this version posted May 9, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. ABSTRACT A prerequisite for the modeling and understanding of the inner ear mechanics needs the accurate created membranous labyrinth. I present a semi-automated methodology for accurate reconstruction of the membranous labyrinth in vivo from high-resolution temporal bone CT data of normal human subjects. -

Characteristic Anatomical Structures of Rat Temporal Bone
HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Otology 10 (2015) 118e124 www.journals.elsevier.com/journal-of-otology/ Characteristic anatomical structures of rat temporal bone Peng Li a,b, Kelei Gao b,c, Dalian Ding a,b,c,*, Richard Salvi b,c a Department of Otolaryngology, Head and Neck Surgery, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, China b Center for Hearing and Deafness, State University of New York at Buffalo, Buffalo, NY 14214, USA c Department of Otolaryngology, Head and Neck Surgery, Xiangya Hospital, Central South University, Hunan 410013, China Abstract As most gene sequences and functional structures of internal organs in rats have been well studied, rat models are widely used in experi- mental medical studies. A large number of descriptions and atlas of the rat temporal bone have been published, but some detailed anatomy of its surface and inside structures remains to be studied. By focusing on some unique characteristics of the rat temporal bone, the current paper aims to provide more accurate and detailed information on rat temporal bone anatomy in an attempt to complete missing or unclear areas in the existed knowledge. We also hope this paper can lay a solid foundation for experimental rat temporal bone surgeries, and promote information exchange among colleagues, as well as providing useful guidance for novice researchers in the field of hearing research involving rats. Copyright © 2015 The Authors. Production & hosting by Elsevier (Singapore) Pte Ltd On behalf of PLA General Hospital Department of Otolaryngology Head and Neck Surgery. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/ by-nc-nd/4.0/). -

Temporal Bone, Normal Anatomy, Computed Tomography/Histology
Temporal Bone Normal Anatomy Computed Tomography /Histology Correlation Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Eric F. Curtin Barbara J. Burgess Dianne D. Jones Jennifer T. O’Malley Hugh D. Curtin MD Otopathologylaboratory.org Facial nerve Malleus Incus Cochlea Lateral canal Oval Window Axial CT Red Arrow -Facial nerve VII Attic IAC Vest LSCC Red Arrow -Facial nerve VII M I M-Malleus I-Incus Vest Cochlea S-Stapes VII S Interscalar septum M M-Modiolus Interscalar septum M M-Modiolus Anterior epitympanic recess Cog Cochleariform process Cochlea Facial recess S-Stapes Pyramidal process S Sinus Tympani ME ME-middle ear RW-round window Cochlea RW Stapedius M Carotid TMJ plate Jugular plate Coronal CT FN 2 FN 1 Cochlea Carotid Malleus Tegmen Incus FN 2 Oval window Scutum Lateral Semicircular Canal Pyramidal process Round Window Jugular Facial Nerve Key image lateral canal facial nerve oval window .. Poschl CT Carotid Tensor tympani Eustachian tube VII1 IAC Basilar turn cochlea Apical turn cochlea Turn 2 cochlea VII1 VII2 Tensor tympani VII2 Jugular vein Tensor tympani Jugular vein Cochleariform process Superior SCC M M-malleus VII2 Oval W Round W Lateral canal Common crus TMJ Lateral SCC VII2 I S I –Incus S- Stapes Tegmen Vestibular aqueduct VII2 VII3 EAC Stenvers CT SSCC VII1 Carotid VII1 is separated from nerve to lateral canal by Bill’s bar Canal for nerve to lateral SCC VII VII1 Basilar turn cochlea LSCC SSCC VII3 Round window Styloid process Vestibule VII3 PSCC Common Crus IAC Axial CT Histology correlation Utricle Lateral Semicircular Canal Vestibule Vestibule Saccule Utricle Vestibule Saccule Utricle Vestibule Saccule Utricle Anterior Crus of the Stapes Carotid Tympanic Carotid plate membrane Middle Ear Jugular Jugular plate Coronal CT Histology correlation Saccule Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Otopathologylaboratory.org Eric F. -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -
B005DTJC8Y.Usermanual.Pdf
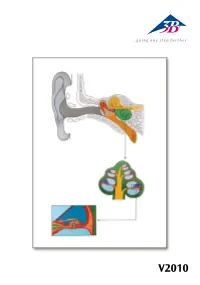
V2010 Auris Latin Auris externa 1 Auricula 2 Meatus acusticus externus 3 Membrana tympanica Auris media 4 Ossicula auditus: 4 a Malleus 4 b Incus 4 c Stapes 5 Cavitas tympani 6 Tuba auditiva 7 Fenestra vestibuli 8 Fenestra cochleae Auris interna 9 Labyrinthus osseus: 9 a Canalis semicircularis posterior 9 b Canalis semicircularis lateralis 9 c Canalis semicircularis anterior 9 d Vestibulum 9 e Cochlea 10 N. vestibularis 11 N. cochlearis ® 12 N. vestibulocochlearis [VIII] 13 Lig. spirale 14 Scala vestibuli 15 Ductus cochlearis 16 Scala tympani 17 N. cochlearis 18 Ganglion cochleare 19 Organum spirale 20 Modiolus cochleae 21 Paries vestibularis 22 Sulcus spiralis internus 23 Membrana tectoria 24 Cuniculus medius 25 Cuniculus externus 26 Cellulae terminales externae 27 Cellulae sustentaculares externae 28 Sulcus spiralis externus 29 Lamina basilaris 30 Cellulae phalangea externae 31 Cellulae capillares externae 32 Cellula stela externa 33 Cuniculus internus 34 Cellula capillaris interna 35 Cellula stela interna 36 Lamina spiralis ossea 37 Limbus spiralis English The Ear A Sectional view of the human ear B Sectional view of cochlea C Sectional view of cochlear duct with spiral organ External ear 1 Auricle 2 External acoustic meatus 3 Tympanic membrane Middle ear 4 Auditory ossicles: 4 a Malleus 4 b Incus 4 c Stapes 5 Tympanic cavity 6 Pharyngotympanice tube 7 Oval window 8 Round window Internal ear 9 Bony labyrinth 9 a Posterior semicircular canal 9 b Lateral semicircular canal 9 c Anterior semicircular canal ® 9 d Vestibule 9 e Cochlea 10 -

Mmubn000001 20756843X.Pdf
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/147581 Please be advised that this information was generated on 2021-10-07 and may be subject to change. CATION TRANSPORT AND COCHLEAR FUNCTION CATION TRANSPORT AND COCHLEAR FUNCTION PROMOTORES: Prof. Dr. S. L. BONTING EN Prof. Dr. W. F. B. BRINKMAN CATION TRANSPORT AND COCHLEAR FUNCTION PROEFSCHRIFT TER VERKRIJGING VAN DE GRAAD VAN DOCTOR IN DE WISKUNDE EN NATUURWETENSCHAPPEN AAN DE KATHOLIEKE UNIVERSITEIT TE NIJMEGEN, OP GEZAG VAN DE RECTOR MAGNIFICUS DR. G. BRENNINKMEIJER, HOOGLERAAR IN DE FACULTEIT DER SOCIALE WETENSCHAPPEN, VOLGENS BESLUIT VAN DE SENAAT IN HET OPENBAAR TE VERDEDIGEN OP VRIJDAG 19 DECEMBER 1969 DES NAMIDDAGS TE 2 UUR DOOR WILLIBRORDUS KUIJPERS GEBOREN TE KLOOSTERZANDE 1969 CENTRALE DRUKKERIJ NIJMEGEN I am greatly indebted to Dr. J. F. G. Siegers for his interest and many valuable discussions throughout the course of this investigation. The technical assistance of Miss A. C. H. Janssen, Mr. A. C. van der Vleuten and Mr. P. Spaan and co-workers was greatly appreciated. I also wish to express my gratitude to Miss. A. E. Gonsalvcs and Miss. G. Kuijpers for typing and to Mrs. M. Duncan for correcting the ma nuscript. The diagrams were prepared by Mr W. Maas and Mr. C. Reckers and the micro- photographs by Mr. A. Reijnen of the department of medical illustration. Aan mijn Ouders, l Thea, Annemarie, Katrien en Michiel. CONTENTS GENERAL INTRODUCTION ... -

Inner Ear Defects and Hearing Loss in Mice Lacking the Collagen Receptor
Laboratory Investigation (2008) 88, 27–37 & 2008 USCAP, Inc All rights reserved 0023-6837/08 $30.00 Inner ear defects and hearing loss in mice lacking the collagen receptor DDR1 Angela M Meyer zum Gottesberge1, Oliver Gross2, Ursula Becker-Lendzian1, Thomas Massing1 and Wolfgang F Vogel3 Discoidin domain receptor 1 (DDR1) is a tyrosine kinase receptor that is activated by native collagen. The physiological functions of DDR1 include matrix homeostasis and cell growth, adhesion, branching, and migration, but the specific role of DDR1 in the development and function of the inner ear has not been analyzed. Here, we show that deletion of the DDR1 gene in mouse is associated with a severe decrease in auditory function and substantial structural alterations in the inner ear. Immunohistochemical analysis demonstrated DDR1 expression in several locations in the cochlea, mostly associated with basement membrane and fibrillar collagens; in particular in basal cells of the stria vascularis, type III fibrocytes, and cells lining the basilar membrane of the organ of Corti. In the stria vascularis, loss of DDR1 function resulted in altered morphology of the basal cells and accumulation of electron-dense matrix within the strial epithelial layer in conjunction with a focal and progressive deterioration of strial cells. Cell types in proximity to the basilar membrane, such as Claudius’, inner and outer sulcus cells, also showed marked ultrastructural alterations. Changes in the organ of Corti, such as deterioration of the supporting cells, specifically the outer hair cells, Deiters’, Hensen’s and bordering cells, are likely to interfere with mechanical properties of the organ and may be responsible for the hearing loss observed in DDR1-null mice. -

Microstructures of the Osseous Spiral Laminae in the Bat Cochlea: a Scanning Electron Microscopic Study
Arch. Histol. Cytol., Vol. 55, No. 3 (1992) p. 315-319 Microstructures of the Osseous Spiral Laminae in the Bat Cochlea: A Scanning Electron Microscopic Study Babi r KUcUK2 and Kazuhiro ABE1 Department of Anatomy, Hokkaido University School of Medicine, Sapporo, Hokkaido; and Department of Otolaryngology2, Tokai University School of Medicine, Isehara, Kanagawa, Japan Received May 22, 1992 Summary. The architecture and surface structures of dary osseous spiral laminae. High frequency sounds the primary and secondary osseous spiral laminae in the vibrate the membrane in the basal regions of the cochlea of the bat, an animal able to hear high fre- cochlear duct, while relatively lower frequency quency sounds, were examined by scanning electron sounds vibrate the membrane in the apical regions microscopy to understand the micromechanical adapta- (BEKESY, 1960) . Using the mouse cochlea, we have tions of the bony supportive elements in the inner ear to suggested that the regional vibration pattern of the the specific hearing function. The bat used was Myotis frater kaguyae. basilar membrane is closely related to the base-to- The myotis bat cochlea was seen to consist of a hook apex variations in the morphology of the osseous and a spiral portion with one and three-quarter turns spiral laminae (KUcUK and ABE, 1989). It is known and was characterized by: 1) a distinct ridge-like pro- that the bat cochlea is sensitive to sounds in the very jection running spirally along the middle line on the high frequency range. In fact, the frequencies of the vestibular leaf of the primary osseous spiral lamina; 2) sounds that stimulate the basilar membrane in the a wide secondary osseous spiral lamina; and 3) a narrow bat cochlea are higher than those that stimulate the spiral fissure between the primary and secondary osse- basal regions of the basilar membrane in the mouse ous spiral laminae. -

Otoancorin, an Inner Ear Protein Restricted To
Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22 Ingrid Zwaenepoel*, Mirna Mustapha*, Michel Leibovici*, Elisabeth Verpy*, Richard Goodyear†, Xue Zhong Liu‡, Sylvie Nouaille*, Walter E. Nance§, Moien Kanaan¶, Karen B. Avrahamʈ, Fredj Tekaia**, Jacques Loiselet††, Marc Lathrop‡‡, Guy Richardson†, and Christine Petit*¶¶ *Unite´deGe´ne´ tique des De´ficits Sensoriels, Centre National de la Recherche Scientifique, Unite´de Recherche Associe´e 1968, and **Unite´deGe´ne´ tique Mole´culaire des Levures, Centre National de la Recherche Scientifique, Unite´de Recherche Associe´e 2171, Institut Pasteur, 25 rue du Dr. Roux, 75724 Paris Cedex 15, France; †School of Biological Sciences, The University of Sussex, Falmer, Brighton, BN1 9QG, United Kingdom; ‡Department of Otolaryngology, University of Miami, Miami, FL 33101; §Department of Human Genetics, Virginia Commonwealth University, 1101 East Marshall Street, Richmond, VA 23219; ¶Molecular Genetics, Life Science Department, Bethlehem University POB 9, Palestinian Authority; ʈDepartment of Human Genetics and Molecular Medicine, Tel Aviv University, Tel Aviv 69978, Israel; ††Laboratoire de Biologie Mole´culaire, Faculte´deMe´ decine, Universite´Saint-Joseph, Beyrouth, Lebanon; and ‡‡Centre National de Ge´notypage, 91057 Evry, France Edited by Thaddeus P. Dryja, Harvard Medical School, Boston, MA, and approved February 21, 2002 (received for review October 1, 2001) A 3,673-bp murine cDNA predicted to encode a glycosylphosphati- collagenase-insensitive striated-sheet matrix (6). Otogelin is asso- dylinositol-anchored protein of 1,088 amino acids was isolated ciated mainly with the collagen fibril bundles (3, 7), whereas ␣- and during a study aimed at identifying transcripts specifically ex- -tectorin are major components of the striated-sheet matrix (8, 9).