Vascular Supply of the Human Spiral Ganglion: Novel Three
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cochlear Partition Anatomy and Motion in Humans Differ from The
Cochlear partition anatomy and motion in humans differ from the classic view of mammals Stefan Raufera,b,1, John J. Guinan Jra,b,c, and Hideko Heidi Nakajimaa,b,c aEaton-Peabody Laboratories, Massachusetts Eye and Ear, Boston, MA 02114; bSpeech and Hearing Bioscience and Technology Program, Harvard University, Cambridge, MA 02138; and cDepartment of Otolaryngology, Harvard Medical School, Boston, MA 02115 Edited by Christopher A. Shera, University of Southern California, Los Angeles, CA, and accepted by Editorial Board Member Thomas D. Albright June 6, 2019 (received for review January 16, 2019) Mammals detect sound through mechanosensitive cells of the assume there is no OSL motion (10–13). Additionally, the attach- cochlear organ of Corti that rest on the basilar membrane (BM). ment of the OSL to the BM and the attachment of the TM to Motions of the BM and organ of Corti have been studied at the spiral limbus (which sits above the OSL) are also consid- the cochlear base in various laboratory animals, and the assump- ered stationary. In contrast to this view, there have been reports tion has been that the cochleas of all mammals work similarly. of sound-induced OSL movement, but these reports have been In the classic view, the BM attaches to a stationary osseous spi- largely ignored in overviews of cochlear mechanics and the for- ral lamina (OSL), the tectorial membrane (TM) attaches to the mation of cochlear models (10–13). Von B´ek´esy (14), using static limbus above the stationary OSL, and the BM is the major mov- pressure, found that the CP “bent like an elastic rod that was free ing element, with a peak displacement near its center. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

Temporal Bone, Normal Anatomy, Computed Tomography/Histology
Temporal Bone Normal Anatomy Computed Tomography /Histology Correlation Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Eric F. Curtin Barbara J. Burgess Dianne D. Jones Jennifer T. O’Malley Hugh D. Curtin MD Otopathologylaboratory.org Facial nerve Malleus Incus Cochlea Lateral canal Oval Window Axial CT Red Arrow -Facial nerve VII Attic IAC Vest LSCC Red Arrow -Facial nerve VII M I M-Malleus I-Incus Vest Cochlea S-Stapes VII S Interscalar septum M M-Modiolus Interscalar septum M M-Modiolus Anterior epitympanic recess Cog Cochleariform process Cochlea Facial recess S-Stapes Pyramidal process S Sinus Tympani ME ME-middle ear RW-round window Cochlea RW Stapedius M Carotid TMJ plate Jugular plate Coronal CT FN 2 FN 1 Cochlea Carotid Malleus Tegmen Incus FN 2 Oval window Scutum Lateral Semicircular Canal Pyramidal process Round Window Jugular Facial Nerve Key image lateral canal facial nerve oval window .. Poschl CT Carotid Tensor tympani Eustachian tube VII1 IAC Basilar turn cochlea Apical turn cochlea Turn 2 cochlea VII1 VII2 Tensor tympani VII2 Jugular vein Tensor tympani Jugular vein Cochleariform process Superior SCC M M-malleus VII2 Oval W Round W Lateral canal Common crus TMJ Lateral SCC VII2 I S I –Incus S- Stapes Tegmen Vestibular aqueduct VII2 VII3 EAC Stenvers CT SSCC VII1 Carotid VII1 is separated from nerve to lateral canal by Bill’s bar Canal for nerve to lateral SCC VII VII1 Basilar turn cochlea LSCC SSCC VII3 Round window Styloid process Vestibule VII3 PSCC Common Crus IAC Axial CT Histology correlation Utricle Lateral Semicircular Canal Vestibule Vestibule Saccule Utricle Vestibule Saccule Utricle Vestibule Saccule Utricle Anterior Crus of the Stapes Carotid Tympanic Carotid plate membrane Middle Ear Jugular Jugular plate Coronal CT Histology correlation Saccule Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Otopathologylaboratory.org Eric F. -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -
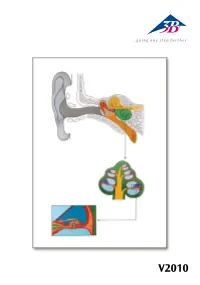
B005DTJC8Y.Usermanual.Pdf
V2010 Auris Latin Auris externa 1 Auricula 2 Meatus acusticus externus 3 Membrana tympanica Auris media 4 Ossicula auditus: 4 a Malleus 4 b Incus 4 c Stapes 5 Cavitas tympani 6 Tuba auditiva 7 Fenestra vestibuli 8 Fenestra cochleae Auris interna 9 Labyrinthus osseus: 9 a Canalis semicircularis posterior 9 b Canalis semicircularis lateralis 9 c Canalis semicircularis anterior 9 d Vestibulum 9 e Cochlea 10 N. vestibularis 11 N. cochlearis ® 12 N. vestibulocochlearis [VIII] 13 Lig. spirale 14 Scala vestibuli 15 Ductus cochlearis 16 Scala tympani 17 N. cochlearis 18 Ganglion cochleare 19 Organum spirale 20 Modiolus cochleae 21 Paries vestibularis 22 Sulcus spiralis internus 23 Membrana tectoria 24 Cuniculus medius 25 Cuniculus externus 26 Cellulae terminales externae 27 Cellulae sustentaculares externae 28 Sulcus spiralis externus 29 Lamina basilaris 30 Cellulae phalangea externae 31 Cellulae capillares externae 32 Cellula stela externa 33 Cuniculus internus 34 Cellula capillaris interna 35 Cellula stela interna 36 Lamina spiralis ossea 37 Limbus spiralis English The Ear A Sectional view of the human ear B Sectional view of cochlea C Sectional view of cochlear duct with spiral organ External ear 1 Auricle 2 External acoustic meatus 3 Tympanic membrane Middle ear 4 Auditory ossicles: 4 a Malleus 4 b Incus 4 c Stapes 5 Tympanic cavity 6 Pharyngotympanice tube 7 Oval window 8 Round window Internal ear 9 Bony labyrinth 9 a Posterior semicircular canal 9 b Lateral semicircular canal 9 c Anterior semicircular canal ® 9 d Vestibule 9 e Cochlea 10 -

Endoscope-Assisted Microsurgical Retrosigmoid Approach to the Lateral Posterior Fossa: Cadaveric Model and a Review of Literature Mohammed A
www.surgicalneurologyint.com Surgical Neurology International Editor-in-Chief: Nancy E. Epstein, MD, Clinical Professor of Neurological Surgery, School of Medicine, State U. of NY at Stony Brook. SNI: Neuroendoscopy Editor J. André Grotenhuis, MD Radboud University Medical Center; Nijmegen, e Netherlands Open Access Review Article Endoscope-assisted microsurgical retrosigmoid approach to the lateral posterior fossa: Cadaveric model and a review of literature Mohammed A. Fouda1, Yasser Jeelani2,3, Abdulkarim Gokoglu2, Rajiv R. Iyer1, Alan R. Cohen1 1Division of Pediatric Neurosurgery, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, 2Department of Neurosurgery, Brigham and Woman’s Hospital, Boston, Massachusetts, 3Department of Neurosurgery, Boston Children’s Hospital, Boston, Massachusetts. E-mail: *Mohammed A. Fouda - [email protected]; Yasser Jeelani - [email protected]; Abdulkarim Gokoglu - [email protected]; Rajiv R. Iyer - [email protected]; Alan R. Cohen - [email protected] ABSTRACT Background: e advancement of endoscopic techniques in the past decade has improved the surgical management of cerebellopontine angle (CPA) tumors. Endoscope-assisted microsurgery improves the ability to evaluate the extent of resection, achieve safe tumor resection and reduce the risk of surgery-related morbidity. Methods: In this study, we used a cadaveric model to demonstrate a step by step endoscope-assisted microsurgery *Corresponding author: of the retrosigmoid approach to the lateral posterior fossa. Mohammed A. Fouda, Division of Pediatric Results: Retrosigmoid craniotomies were performed on four latex-injected cadaver heads (eight CPAs). Microsurgical exposures were performed to identify neurovascular structures in each segment. 0° and 30° rigid Neurosurgery, Department endoscope lenses were subsequently introduced into each corridor and views were compared in this manner. -

Posterior Circulation Stroke
Posterior Circulation Stroke Sheryl Martin-Schild, MD, PhD, FANA, FAHA Stroke Medical Director for Louisiana Emergency Response Network (LERN) Medical Director of Neurology & Stroke – New Orleans East Hospital and Touro Infirmary President & CEO - Dr. Brain, Inc. Webinar #15 Posterior circulation stroke • Review anatomy – pipes, plumbing, & parenchyma • Common stroke syndromes • NIHSS exam - limitations • The 5 D’s – working through ddx • Supplemental examination • Evaluating the acutely vertiginous patient • Evaluating the patient with perceived minor stroke • Advanced imaging - pitfalls • Standard-of-care treatment options Posterior circulation stroke - Pipes Posterior circulation stroke Plumbing variation • Normal Circle of Willis • <50% population Posterior circulation stroke Plumbing variation Fetal PCA • fPCA (9.5%) is continuation of Pcomm • No communication with basilar • Partial fPCA (15%) has atretic communication with basilar artery • lack of or smaller thalamoperforators in the absence of a P1 or atretic P1 Posterior circulation stroke Plumbing variation • Dominant VA 2/3 • Persistent trigeminal artery • PCA = midbrain, thalamus, medial surface of occipital lobe, inferior and medial surfaces of temporal lobe • SCA = superior cerebellum & rostral laterodorsal pons • AICA = lateral caudal pons & part of cerebellum • PICA = lateral medulla & inferior cerebellum Common stroke syndromes associated with vessel occlusions • Posterior cerebral artery • Basilar artery • Superior cerebellar artery • Anterior inferior cerebellar artery -

Anomalies of Basilar Artery and Variations of Its
Int J Biol Med Res.2018 ;9(1):6200-6204 Int J Biol Med Res www.biomedscidirect.com Volume 6, Issue 2, April 2015 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research Journal homepage: www.biomedscidirect.com BioMedSciDirect International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Original Article ANOMALIES OF BASILAR ARTERY AND VARIATIONS OF ITS BRANCHES WITH SURGICAL PERSPECTIVES Dr.Sreenivas Reddy.K a, Dr.Chavalin V Bharath b Assistant Professor of Anatomy, KLR Lenora Institute of Dental Sciences, NH16, Rajanagaram, Rajahmundry – 533 294, East Godavari (Dist.), A.P. India. Assistant Professor of Anatomy, Alluri Sitaramaraju Academy of Medical Sciences, Eluru, 534005, West Godavari District, Andhra Pradesh, India. A R T I C L E I N F O A B S T R A C T Keywords: ABSTRACT: The basilar artery is formed by the union of two vertebral arteries and it forms an basilar artery important part of the posterior circulation of the brain. The present study is based on the fevibond acetone solution stenosis observation of brain specimens obtained from the cadavers and specimens were preserved in aneurysm 10% formalin for 1 month. The fevibond - acetone solution enabled to fix up and gave a shining smooth surface for further painting. Many vascular bypass and shunting procedures for the treatment of stenosis, occlusions, aneurysms, arterio-venous malformations of the arteries of the posterior cranial fossa. Anatomical variations, duplications, fenestration or the absence of certain vessel will significantly alter the plan of surgical approach and also determines the outcome of revascularization procedures. Hence a detailed anatomical knowledge is necessary for successful design and completion of any surgical procedures. -

Microsurgical Anatomy of the Dural Arteries
ANATOMIC REPORT MICROSURGICAL ANATOMY OF THE DURAL ARTERIES Carolina Martins, M.D. OBJECTIVE: The objective was to examine the microsurgical anatomy basic to the Department of Neurological microsurgical and endovascular management of lesions involving the dural arteries. Surgery, University of Florida, Gainesville, Florida METHODS: Adult cadaveric heads and skulls were examined using the magnification provided by the surgical microscope to define the origin, course, and distribution of Alexandre Yasuda, M.D. the individual dural arteries. Department of Neurological RESULTS: The pattern of arterial supply of the dura covering the cranial base is more Surgery, University of Florida, complex than over the cerebral convexity. The internal carotid system supplies the Gainesville, Florida midline dura of the anterior and middle fossae and the anterior limit of the posterior Alvaro Campero, M.D. fossa; the external carotid system supplies the lateral segment of the three cranial Department of Neurological fossae; and the vertebrobasilar system supplies the midline structures of the posterior Surgery, University of Florida, fossa and the area of the foramen magnum. Dural territories often have overlapping Gainesville, Florida supply from several sources. Areas supplied from several overlapping sources are the parasellar dura, tentorium, and falx. The tentorium and falx also receive a contribution Arthur J. Ulm, M.D. from the cerebral arteries, making these structures an anastomotic pathway between Department of Neurological Surgery, University of Florida, the dural and parenchymal arteries. A reciprocal relationship, in which the territories Gainesville, Florida of one artery expand if the adjacent arteries are small, is common. CONCLUSION: The carotid and vertebrobasilar arterial systems give rise to multiple Necmettin Tanriover, M.D. -

Appleton & Lange Review of Anatomy
0523-00 FM 07/15/02 15:30 Page i Sixth edition APPLETON & LANGE REVIEW OF ANATOMY Royce Lee Montgomery, PhD Professor Department of Cell and Developmental Biology School of Medicine University of North Carolina Chapel Hill, North Carolina Kurt Ogden Gilliland, PhD Department of Cell and Developmental Biology School of Medicine University of North Carolina Chapel Hill, North Carolina Appleton & Lange Reviews/McGraw-Hill Medical Publishing Division New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto 0523-00 FM 07/15/02 15:30 Page ii Appleton & Lange Review of Anatomy, Sixth Edition Copyright © 2003 by The McGraw-Hill Companies, Inc. All rights reserved. Printed in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a data base or retrieval system, without the prior written permission of the publisher. Previous editions copyright © 1995, 1989, by Appleton & Lange; copyright © 1982, 1978, 1974, by Arco Publishing, Inc. 1 2 3 4 5 6 7 8 9 0 VNH VNH 0 9 8 7 6 5 4 3 2 ISBN: 0-07-137727-1 Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the stan- dards accepted at the time of publication. -

Management of Anterior Inferior Cerebellar Artery Aneurysms: an Illustrative Case and Review of Literature
Neurosurg Focus 26 (5):E6, 2009 Management of anterior inferior cerebellar artery aneurysms: an illustrative case and review of literature NICHOLAS C. BAM B AKIDIS , M.D.,1 SU N IL MA N JILA , M.D.,1 SHERVI N DASHTI , M.D.,2 RO B ERT TARR , M.D.,3 A N D CLIFF A. MEGERIA N , M.D.4 1Department of Neurological Surgery, University Hospitals Case Medical Center, Cleveland, Ohio; 2Division of Neurosurgery, Barrow Neurological Institute, Phoenix, Arizona; and Departments of 3Neuroradiology and 4Otolaryngology–Head and Neck Surgery, University Hospitals Case Medical Center, Cleveland, Ohio Aneurysms of the anterior inferior cerebellar artery (AICA) are relatively rare among intracranial aneurysms. They can occur in 1 of 3 regions of the AICA: 1) craniocaudal (high or low riding), 2) mediolateral-premeatal (proxi- mal), and 3) meatal-postmeatal (distal). The management strategies for treatment differ according to the location and configuration of the aneurysm. The existing body of neurosurgical literature contains articles published on aneurysms arising from the AICA near the basilar artery (BA), intracanalicular/meatal aneurysms, and distal AICA. Several therapeutic options exist, encompassing microsurgical and endovascular techniques. The authors describe a case of treatment involving a large BA-AICA aneurysm approached via exposure of the presigmoid dura using a retromas- toid suboccipital craniectomy and partial petrosectomy. Treatment of these lesions requires detailed knowledge of the anatomy, and an anatomical overview of the AICA with its arterial loops and significant branches is presented, including a discussion of the internal auditory (labyrinthine) artery, recurrent perforating arteries, subarcuate artery, and cerebellosubarcuate artery. The authors discuss the various surgical approaches (retromastoid, far lateral, subtem- poral, and transclival) with appropriate illustrations, citing the advantages and disadvantages in accessing these AICA lesions in relation to these approaches.