Dynamic Patterns of Neurotrophin 3 Expression in the Postnatal Mouse Inner Ear
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reticular Lamina and Basilar Membrane Vibrations in Living Mouse Cochleae
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Rena,1, Wenxuan Hea, and David Kempb aOregon Hearing Research Center, Department of Otolaryngology, Oregon Health & Science University, Portland, OR 97239; and bUniversity College London Ear Institute, University College London, London WC1E 6BT, United Kingdom Edited by Mario A. Ruggero, Northwestern University, Evanston, IL, and accepted by Editorial Board Member Peter L. Strick July 1, 2016 (received for review May 9, 2016) It is commonly believed that the exceptional sensitivity of mamma- (Fig. 1 A and B and Materials and Methods). The results from this lian hearing depends on outer hair cells which generate forces for study do not demonstrate the commonly expected cochlear local amplifying sound-induced basilar membrane vibrations, yet how feedback but instead indicate a global hydromechanical mecha- cellular forces amplify vibrations is poorly understood. In this study, nism for outer hair cells to enhance hearing sensitivity. by measuring subnanometer vibrations directly from the reticular lamina at the apical ends of outer hair cells and from the basilar Results membrane using a custom-built heterodyne low-coherence interfer- Vibrations of the Reticular Lamina and Basilar Membrane in Sensitive ometer, we demonstrate in living mouse cochleae that the sound- Cochleae. Vibrations of the reticular lamina and basilar membrane induced reticular lamina vibration is substantially larger than the measured from a sensitive cochlea are presented in Fig. 1 C–J. basilar membrane vibration not only at the best frequency but Below 50 dB sound pressure level (SPL) (0 dB SPL = 20 μPa), the surprisingly also at low frequencies. -

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Auditory Neuropathy After Damage to Cochlear Spiral Ganglion Neurons
www.nature.com/scientificreports OPEN Auditory Neuropathy after Damage to Cochlear Spiral Ganglion Neurons in Mice Resulting from Conditional Received: 27 July 2016 Accepted: 15 June 2017 Expression of Diphtheria Toxin Published online: 25 July 2017 Receptors Haolai Pan1, Qiang Song1, Yanyan Huang1, Jiping Wang1, Renjie Chai2, Shankai Yin1 & Jian Wang 1,3 Auditory neuropathy (AN) is a hearing disorder characterized by normal cochlear amplifcation to sound but poor temporal processing and auditory perception in noisy backgrounds. These defcits likely result from impairments in auditory neural synchrony; such dyssynchrony of the neural responses has been linked to demyelination of auditory nerve fbers. However, no appropriate animal models are currently available that mimic this pathology. In this study, Cre-inducible diphtheria toxin receptor (iDTR+/+) mice were cross-mated with mice containing Cre (Bhlhb5-Cre+/−) specifc to spiral ganglion neurons (SGNs). In double-positive ofspring mice, the injection of diphtheria toxin (DT) led to a 30–40% rate of death for SGNs, but no hair cell damage. Demyelination types of pathologies were observed around the surviving SGNs and their fbers, many of which were distorted in shape. Correspondingly, a signifcant reduction in response synchrony to amplitude modulation was observed in this group of animals compared to the controls, which had a Cre− genotype. Taken together, our results suggest that SGN damage following the injection of DT in mice with Bhlhb5-Cre+/− and iDTR+/− is likely to be a good AN model of demyelination. Auditory neuropathy (AN) is a hearing disorder characterized as having normal cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), but largely reduced or missing auditory brainstem responses (ABRs). -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Cochlear Partition Anatomy and Motion in Humans Differ from The
Cochlear partition anatomy and motion in humans differ from the classic view of mammals Stefan Raufera,b,1, John J. Guinan Jra,b,c, and Hideko Heidi Nakajimaa,b,c aEaton-Peabody Laboratories, Massachusetts Eye and Ear, Boston, MA 02114; bSpeech and Hearing Bioscience and Technology Program, Harvard University, Cambridge, MA 02138; and cDepartment of Otolaryngology, Harvard Medical School, Boston, MA 02115 Edited by Christopher A. Shera, University of Southern California, Los Angeles, CA, and accepted by Editorial Board Member Thomas D. Albright June 6, 2019 (received for review January 16, 2019) Mammals detect sound through mechanosensitive cells of the assume there is no OSL motion (10–13). Additionally, the attach- cochlear organ of Corti that rest on the basilar membrane (BM). ment of the OSL to the BM and the attachment of the TM to Motions of the BM and organ of Corti have been studied at the spiral limbus (which sits above the OSL) are also consid- the cochlear base in various laboratory animals, and the assump- ered stationary. In contrast to this view, there have been reports tion has been that the cochleas of all mammals work similarly. of sound-induced OSL movement, but these reports have been In the classic view, the BM attaches to a stationary osseous spi- largely ignored in overviews of cochlear mechanics and the for- ral lamina (OSL), the tectorial membrane (TM) attaches to the mation of cochlear models (10–13). Von B´ek´esy (14), using static limbus above the stationary OSL, and the BM is the major mov- pressure, found that the CP “bent like an elastic rod that was free ing element, with a peak displacement near its center. -

Vibration Hotspots Reveal Longitudinal Funneling of Sound-Evoked Motion in the Mammalian Cochlea
ARTICLE DOI: 10.1038/s41467-018-05483-z OPEN Vibration hotspots reveal longitudinal funneling of sound-evoked motion in the mammalian cochlea Nigel P. Cooper 1, Anna Vavakou1 & Marcel van der Heijden 1 The micromechanical mechanisms that underpin tuning and dynamic range compression in the mammalian inner ear are fundamental to hearing, but poorly understood. Here, we present new, high-resolution optical measurements that directly map sound-evoked vibra- 1234567890():,; tions on to anatomical structures in the intact, living gerbil cochlea. The largest vibrations occur in a tightly delineated hotspot centering near the interface between the Deiters’ and outer hair cells. Hotspot vibrations are less sharply tuned, but more nonlinear, than basilar membrane vibrations, and behave non-monotonically (exhibiting hyper-compression) near their characteristic frequency. Amplitude and phase differences between hotspot and basilar membrane responses depend on both frequency and measurement angle, and indicate that hotspot vibrations involve longitudinal motion. We hypothesize that structural coupling between the Deiters’ and outer hair cells funnels sound-evoked motion into the hotspot region, under the control of the outer hair cells, to optimize cochlear tuning and compression. 1 Department of Neuroscience, Erasmus MC, Room Ee 1285, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands. Correspondence and requests for materials should be addressed to M.v.d.H. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:3054 | DOI: 10.1038/s41467-018-05483-z -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -
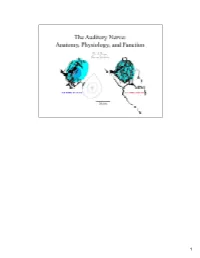
Auditory Nerve.Pdf
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural signals representing these features are carried into the brain by the auditory nerve. It is thought that features of the sounds are processed centrally along parallel and hierarchical pathways where eventually percepts of the sounds are organized. 2 In mammals, the neural representation of acoustic information enters the brain by way of the auditory nerve. The auditory nerve terminates in the cochlear nucleus, and the cochlear nucleus in turn gives rise to multiple output projections that form separate but parallel limbs of the ascending auditory pathways. How the brain normally processes acoustic information will be heavily dependent upon the organization of auditory nerve input to the cochlear nucleus and on the nature of the different neural circuits that are established at this early stage. 3 This histology slide of a cat cochlea (right) illustrates the sensory receptors, the auditory nerve, and its target the cochlear nucleus. The orientation of the cut is illustrated by the pink line in the drawing of the cat head (left). We learned about the relationship between these structures by inserting a dye-filled micropipette into the auditory nerve and making small injections of the dye. After histological processing, stained single fibers were reconstruct back to their origin, and traced centrally to determine how they terminated in the brain. We will review the components of the nerve with respect to composition, innervation of the receptors, cell body morphology, myelination, and central terminations. -

Ultrastructural Analysis and ABR Alterations in the Cochlear Hair-Cells Following Aminoglycosides Administration in Guinea Pig
Global Journal of Otolaryngology ISSN 2474-7556 Research Article Glob J Otolaryngol Volume 15 Issue 4 - May 2018 Copyright © All rights are reserved by Mohammed A Akeel DOI: 10.19080/GJO.2018.15.555916 Ultrastructural Analysis and ABR Alterations in the Cochlear Hair-cells Following Aminoglycosides Administration in Guinea Pig Mohammed A Akeel* Department of Anatomy, Faculty of Medicine, Jazan University, Jazan, Kingdom of Saudi Arabia Submission: April 18, 2018; Published: May 08, 2018 *Corresponding author: Mohammed A Akeel, Faculty of Medicine, Jazan University, P.O.Box 114, Jazan, Kingdom of Saudi Arabia. Tel: ; Email: Abstract Aims: since their introduction in the 1940. The aim of the present work was to characterize ultrastructural alterations of the sensory hair-cell following different aminoglycosides Aminoglycosides administration, (AG) antibiotics intratympanic were the first VS ototoxic intraperitoneal agents to and highlight to correlate the problem them with of drug-induced auditory brainstem hearing responses and vestibular (ABR). loss, Methods: streptomycin, gentamycin, or netilmicin; respectively, via the peritoneal route. The next four groups received similar treatment via trans- tympanic route. A Thetotal treatment of 48 adult was guinea administered pigs were dividedfor seven into consecutive 8 groups; sixdays. animals On day in 10,each ABR group. was The utilized first fourfor hearing groups receivedevaluation saline and (control),scanning electron microscopy (SEM) examination of the sensory organs was used for morphological study. Results: Streptomycin produced the most severe morphological changes and a higher elevation of ABR thresholds, followed, in order, by gentamycin and netilmicin. Netilmicin ototoxicity observed in both systemic and transtympanic routes was low because of lesser penetration into the inner ear or/ and lower intrinsic toxicity. -

The Tectorial Membrane of the Rat'
The Tectorial Membrane of the Rat’ MURIEL D. ROSS Department of Anatomy, The University of Michigan, Ann Arbor, Michigan 48104 ABSTRACT Histochemical, x-ray analytical and scanning and transmission electron microscopical procedures have been utilized to determine the chemical nature, physical appearance and attachments of the tectorial membrane in nor- mal rats and to correlate these results with biochemical data on protein-carbo- hydrate complexes. Additionally, pertinent histochemical and ultrastructural findings in chemically sympathectomized rats are considered. The results indi- cate that the tectorial membrane is a viscous, complex, colloid of glycoprotein( s) possessing some oriented molecules and an ionic composition different from either endolymph or perilymph. It is attached to the reticular laminar surface of the organ of Corti and to the tips of the outer hair cells; it is attached to and encloses the hairs of the inner hair cells. A fluid compartment may exist within the limbs of the “W’formed by the hairs on each outer hair cell surface. Present biochemical concepts of viscous glycoproteins suggest that they are polyelectro- lytes interacting physically to form complex networks. They possess character- istics making them important in fluid and ion transport. Furthermore, the macro- molecular configuration assumed by such polyelectrolytes is unstable and subject to change from stress or shifts in pH or ions. Thus, the attachments of the tec- torial membrane to the hair cells may play an important role in the transduction process at the molecular level. The present investigation is an out- of the tectorial membrane remain matters growth of a prior study of the effects of of dispute.