A Place Principle for Vertigo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Auditory Neuropathy After Damage to Cochlear Spiral Ganglion Neurons
www.nature.com/scientificreports OPEN Auditory Neuropathy after Damage to Cochlear Spiral Ganglion Neurons in Mice Resulting from Conditional Received: 27 July 2016 Accepted: 15 June 2017 Expression of Diphtheria Toxin Published online: 25 July 2017 Receptors Haolai Pan1, Qiang Song1, Yanyan Huang1, Jiping Wang1, Renjie Chai2, Shankai Yin1 & Jian Wang 1,3 Auditory neuropathy (AN) is a hearing disorder characterized by normal cochlear amplifcation to sound but poor temporal processing and auditory perception in noisy backgrounds. These defcits likely result from impairments in auditory neural synchrony; such dyssynchrony of the neural responses has been linked to demyelination of auditory nerve fbers. However, no appropriate animal models are currently available that mimic this pathology. In this study, Cre-inducible diphtheria toxin receptor (iDTR+/+) mice were cross-mated with mice containing Cre (Bhlhb5-Cre+/−) specifc to spiral ganglion neurons (SGNs). In double-positive ofspring mice, the injection of diphtheria toxin (DT) led to a 30–40% rate of death for SGNs, but no hair cell damage. Demyelination types of pathologies were observed around the surviving SGNs and their fbers, many of which were distorted in shape. Correspondingly, a signifcant reduction in response synchrony to amplitude modulation was observed in this group of animals compared to the controls, which had a Cre− genotype. Taken together, our results suggest that SGN damage following the injection of DT in mice with Bhlhb5-Cre+/− and iDTR+/− is likely to be a good AN model of demyelination. Auditory neuropathy (AN) is a hearing disorder characterized as having normal cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), but largely reduced or missing auditory brainstem responses (ABRs). -
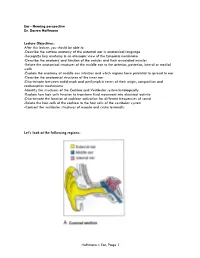
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Anatomy of the Superior Olivary Complex.Pdf
Douglas Oliver University of Connecticut Health Center SUPERIOR OLIVE Auditory Pathways Auditory CORTEX GLUT Cortex GABA GLY Medial Geniculate MGB Body Inferior IC Colliculus DLL DLL COCHLEA VLL VLL DCN VCN SOC Auditory Pathways IC Organization of Superior Olivary Complex . Subdivisions and Cytoarchitecture . Neuron types . Inputs . Outputs . Synapses . Basic Circuit Cytoarchitecture of Superior Olivary Complex LSO LSO MSO MSO MNTB D MNTB M (somata & dendrites) (axons & endings) Tsuchitani, 1978, Fig. 10 Comparative anatomy of SOC Tetsufumi Ito & Shig Kuwada Binaural Basic Circuits 8 ‐ 9 Brodal Fig MSO: medial superior olive; LSO: lateral superior olive NTB: nucleus of trapezoid body; IC: inferior colliculus MSO Principle glutamate Cells . Fusiform . Bipolar . Disc‐shaped . Each dendrite innervated by a different side MSO‐In situ hybridization RPO MSO MNTB SPO LSO VGLUT1 VGLUT2 VIAAT NISSL MSO Inputs and Synapses H=high frequency EI - ILD L=low frequency EE - ITD LSO MSO L L B H B B H G LNTB TO LSO MNTB E=Excitation (glutamate) ‐‐‐ I=Inhibition (glycine) ITD CODING Unlike retinal targets, the cochlear nuclei contain maps of frequency, not location. So how does the auditory system know ‘where’ a sound is coming from? T + ITD T By comparing the interaural time differences (ITD) between the ears How is this accomplished?... LSO MSO Right Input A Right Input B C Time Code Time Code E E A A B B C C D D E E Output Output abcde Place Code abcde Place Code Excitation MSO creates a response to Left Input Left Input Inhibition interaural time differences I Time Code E Time Code DEMSO "peak" unit LSO "trough" unit ITD ITD Figure 14.2 Binaural Responses in MSO MSO Summary . -

The Superior Olivary Complex +
Excitatory and inhibitory transmission in the superior olivary complex. Ian D. Forsythe, Matt Barker, Margaret Barnes-Davies, Brian Billups, Paul Dodson, Fatima Osmani, Steven Owens and Adrian Wong. Department of Cell Physiology and Pharmacology, University of Leicester, Leicester LE1 9HN. UK. The timing and pattern of action potentials propagating into the brainstem from both cochleae contain information about the azimuth location of that sound in auditory space. This binaural information is integrated in the superior olivary complex. This part of the auditory pathway is adapted for fast conduction speeds and the preservation of timing information with several complimentary mechanisms (see Oertel, 1999; Trussell, 1999). There are large diameter axons terminating in giant somatic synapses that activate receptor ion channels with fast kinetics. The resultant postsynaptic potentials generated in the receiving neuron are integrated with a suite of voltage-gated ion channels that determine the action potential threshold, duration and repetitive firing properties. We have studied presynaptic and postsynaptic mechanisms that regulate efficacy, timing and integration of synaptic responses in the medial nucleus of the trapezoid body and the medial and lateral superior olives. Presynaptic calcium currents in the calyx of Held. The calyx of Held is a giant synaptic terminal that forms around the soma of principal cells in the Medial Nucleus of the Trapezoid Body (MNTB) (Forsythe, 1994). Each MNTB neuron receives a single calyx. Action potentials propagating into the synaptic terminal trigger the opening of P-type calcium channels (Forsythe et al. 1998) which in turn trigger the release of glutamate into the synaptic cleft (Borst et al., 1995). -

Use of Calcium-Binding Proteins to Map Inputs in Vestibular Nuclei of the Gerbil
THE JOURNAL OF COMPARATIVE NEUROLOGY 386:317–327 (1997) Use of Calcium-Binding Proteins to Map Inputs in Vestibular Nuclei of the Gerbil GOLDA ANNE KEVETTER* AND ROBERT B. LEONARD Departments of Otolaryngology, Anatomy and Neurosciences, and Physiology and Biophysics, University of Texas Medical Branch, Galveston, Texas 77555 ABSTRACT We wished to determine whether calbindin and/or calretinin are appropriate markers for vestibular afferents, a population of neurons in the vestibular nuclear complex, or cerebellar Purkinje inputs. To accomplish this goal, immunocytochemical staining was observed in gerbils after lesions of the vestibular nerve central to the ganglion, the cerebellum, or both. Eleven to fourteen days after recovery, the brain was processed for immunocytochemical identification of calretinin and calbindin. After lesion of the vestibular nerve, no calretinin staining was seen in any of the vestibular nuclei except for a population of intrinsic neurons, which showed no obvious change in number or staining pattern. Calbindin staining was reduced in all nuclei except the dorsal part of the lateral vestibular nuclei. The density of staining of each marker, measured in the magnocellular medial vestibular nucleus, was signifi- cantly reduced. After the cerebellar lesion, no differences in calretinin staining were noted. However, calbindin staining was greatly reduced in all nuclei. The density of staining, measured in the caudal medial vestibular nucleus, was significantly lower. After a combined lesion of the cerebellum and vestibular nerve, the distribution and density of calretinin staining resembled that after vestibular nerve section alone, whereas calbindin staining was no longer seen. This study demonstrates that calretinin and calbindin are effective markers for the identification of vestibular afferents. -

Cranial Nerves 1, 5, 7-12
Cranial Nerve I Olfactory Nerve Nerve fiber modality: Special sensory afferent Cranial Nerves 1, 5, 7-12 Function: Olfaction Remarkable features: – Peripheral processes act as sensory receptors (the other special sensory nerves have separate Warren L Felton III, MD receptors) Professor and Associate Chair of Clinical – Primary afferent neurons undergo continuous Activities, Department of Neurology replacement throughout life Associate Professor of Ophthalmology – Primary afferent neurons synapse with secondary neurons in the olfactory bulb without synapsing Chair, Division of Neuro-Ophthalmology first in the thalamus (as do all other sensory VCU School of Medicine neurons) – Pathways to cortical areas are entirely ipsilateral 1 2 Crania Nerve I Cranial Nerve I Clinical Testing Pathology Anosmia, hyposmia: loss of or impaired Frequently overlooked in neurologic olfaction examination – 1% of population, 50% of population >60 years Aromatic stimulus placed under each – Note: patients with bilateral anosmia often report nostril with the other nostril occluded, eg impaired taste (ageusia, hypogeusia), though coffee, cloves, or soap taste is normal when tested Note that noxious stimuli such as Dysosmia: disordered olfaction ammonia are not used due to concomitant – Parosmia: distorted olfaction stimulation of CN V – Olfactory hallucination: presence of perceived odor in the absence of odor Quantitative clinical tests are available: • Aura preceding complex partial seizures of eg, University of Pennsylvania Smell temporal lobe origin -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner.