Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Independence of the Endovestibular Potential in Homeotherms
Independence of the Endovestibular Potential in Homeotherms ROBERT S. SCHMIDT From the Department of Surgery (Otolaryngology), University of Chicago, Chicago ABS TR Ac T The endolymphatic potential was recorded from various vestibular parts of the labyrinth from which the cochlea (in the case of guinea pigs) or the cochlea, lagena, and sacculus (in the case of pigeons) had been removed. This endovesfibular potential of the isolated vestibule declined during anoxia and recovered after anoxia in the same manner as the endovestibular potential of the intact labyrinth. Its non-anoxic level was the same as in the intact laby- rinth; i.e., +5 to -[-8 mv in the pigeon and +2 to +5 mv in the guinea pig. It is, therefore, concluded that the endovestibular potential is independent of the cochlea, stria vascularis, and endocochlear potential. INTRODUCTION The endolymphatic potential discovered by B~k~sy (1) has been studied in both the cochlea (2) and vestibule (3, 4). This potential in the cochlea, the endocochlear potential (ECP), is about 80 mv positive in mammals and about 15 mv positive in birds (5). The potential in the vestibular parts of the labyrinth, the endovestibular potential (EVP), is much lower in homeotherms (4--6). Three assumptions regarding the EVP are quite common (4, 7, 8):--that nothing compared to the stria vascularis, the probable source of the ECP, is found in the vestibule; that the EVP results merely from spread of the ECP; and that the EVP is therefore of little interest or importance. These assumptions have very little theoretical or experimental foundation. -
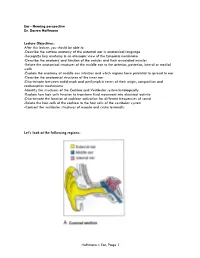
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Endolymphatic Hydrops Meniere's
ENDOLYMPHATIC HYDROPS MENIERE’S DISEASE Introduction: Meniere’s Disease, or endolymphatic hydrops, is a disorder of the inner ear. This condition occurs because of abnormal fluctuations in the inner ear fluid called endolymph. A system of membranes, called the membranous labyrinth, contains a fluid called endolymph. This fluid bathes the inner ear balance and hearing system sensory cells and allows them to function normally. The membranes can become dilated like a balloon when pressure increases. This is called "hydrops". The amount of fluid is normally kept constant by altering the production and absorption of the fluid. Endolymph also contains a specific concentration of sodium, potassium, chloride, and other electrolytes. If the inner ear is damaged by disease, injury, or other causes, the volume and composition of the inner ear fluid can fluctuate with changes in the body’s fluid and electrolyte levels. This fluctuation in inner ear fluid can cause the symptoms of hydrops, including pressure or fullness of the affected ear, tinnitus, hearing loss, and imbalance or dizziness. Treatment of this condition is geared towards stabilizing the body fluid levels so that fluctuations in the endolymph volume can be avoided. Meniere's episodes may occur in clusters in which several attacks may occur within a short period of time. On the other hand, years may pass between episodes. Between the acute attacks, most people are free of symptoms or note mild imbalance, tinnitus, and/or hearing loss. Meniere’s affects roughly 0.2% of the population, about 200 out of 100,000 people (or in other words, 2/1000). -

Identification of a 275-Kd Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell
Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell To cite this version: G Richardson, Sylvain Bartolami, I Russell. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear. Journal of Cell Biology, Rockefeller University Press, 1990, 110, 10.1083/jcb.110.4.1055. hal-02156410 HAL Id: hal-02156410 https://hal.archives-ouvertes.fr/hal-02156410 Submitted on 18 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G. P. Richardson, S. Bartolami, and I. J. Russell School of Biological Sciences, University of Sussex, Falmer, Brighton, United Kingdom Abstract. Immunological techniques have been used basilar papilla (an auditory organ) only the proximal to generate both polyclonal and monoclonal antibodies region of the stereocilia bundle nearest to the apical specific for the apical ends of sensory hair cells in the surface is stained. The monoclonal anti-hair cell anti- avian inner ear. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

The Role of Potassium Recirculation in Cochlear Amplification
The role of potassium recirculation in cochlear amplification Pavel Mistrika and Jonathan Ashmorea,b aUCL Ear Institute and bDepartment of Neuroscience, Purpose of review Physiology and Pharmacology, UCL, London, UK Normal cochlear function depends on maintaining the correct ionic environment for the Correspondence to Jonathan Ashmore, Department of sensory hair cells. Here we review recent literature on the cellular distribution of Neuroscience, Physiology and Pharmacology, UCL, Gower Street, London WC1E 6BT, UK potassium transport-related molecules in the cochlea. Tel: +44 20 7679 8937; fax: +44 20 7679 8990; Recent findings e-mail: [email protected] Transgenic animal models have identified novel molecules essential for normal hearing Current Opinion in Otolaryngology & Head and and support the idea that potassium is recycled in the cochlea. The findings indicate that Neck Surgery 2009, 17:394–399 extracellular potassium released by outer hair cells into the space of Nuel is taken up by supporting cells, that the gap junction system in the organ of Corti is involved in potassium handling in the cochlea, that the gap junction system in stria vascularis is essential for the generation of the endocochlear potential, and that computational models can assist in the interpretation of the systems biology of hearing and integrate the molecular, electrical, and mechanical networks of the cochlear partition. Such models suggest that outer hair cell electromotility can amplify over a much broader frequency range than expected from isolated cell studies. Summary These new findings clarify the role of endolymphatic potassium in normal cochlear function. They also help current understanding of the mechanisms of certain forms of hereditary hearing loss. -

Anatomy of the Ear ANATOMY & Glossary of Terms
Anatomy of the Ear ANATOMY & Glossary of Terms By Vestibular Disorders Association HEARING & ANATOMY BALANCE The human inner ear contains two divisions: the hearing (auditory) The human ear contains component—the cochlea, and a balance (vestibular) component—the two components: auditory peripheral vestibular system. Peripheral in this context refers to (cochlea) & balance a system that is outside of the central nervous system (brain and (vestibular). brainstem). The peripheral vestibular system sends information to the brain and brainstem. The vestibular system in each ear consists of a complex series of passageways and chambers within the bony skull. Within these ARTICLE passageways are tubes (semicircular canals), and sacs (a utricle and saccule), filled with a fluid called endolymph. Around the outside of the tubes and sacs is a different fluid called perilymph. Both of these fluids are of precise chemical compositions, and they are different. The mechanism that regulates the amount and composition of these fluids is 04 important to the proper functioning of the inner ear. Each of the semicircular canals is located in a different spatial plane. They are located at right angles to each other and to those in the ear on the opposite side of the head. At the base of each canal is a swelling DID THIS ARTICLE (ampulla) and within each ampulla is a sensory receptor (cupula). HELP YOU? MOVEMENT AND BALANCE SUPPORT VEDA @ VESTIBULAR.ORG With head movement in the plane or angle in which a canal is positioned, the endo-lymphatic fluid within that canal, because of inertia, lags behind. When this fluid lags behind, the sensory receptor within the canal is bent. -

The Tectorial Membrane of the Rat'
The Tectorial Membrane of the Rat’ MURIEL D. ROSS Department of Anatomy, The University of Michigan, Ann Arbor, Michigan 48104 ABSTRACT Histochemical, x-ray analytical and scanning and transmission electron microscopical procedures have been utilized to determine the chemical nature, physical appearance and attachments of the tectorial membrane in nor- mal rats and to correlate these results with biochemical data on protein-carbo- hydrate complexes. Additionally, pertinent histochemical and ultrastructural findings in chemically sympathectomized rats are considered. The results indi- cate that the tectorial membrane is a viscous, complex, colloid of glycoprotein( s) possessing some oriented molecules and an ionic composition different from either endolymph or perilymph. It is attached to the reticular laminar surface of the organ of Corti and to the tips of the outer hair cells; it is attached to and encloses the hairs of the inner hair cells. A fluid compartment may exist within the limbs of the “W’formed by the hairs on each outer hair cell surface. Present biochemical concepts of viscous glycoproteins suggest that they are polyelectro- lytes interacting physically to form complex networks. They possess character- istics making them important in fluid and ion transport. Furthermore, the macro- molecular configuration assumed by such polyelectrolytes is unstable and subject to change from stress or shifts in pH or ions. Thus, the attachments of the tec- torial membrane to the hair cells may play an important role in the transduction process at the molecular level. The present investigation is an out- of the tectorial membrane remain matters growth of a prior study of the effects of of dispute. -

The Temporal Bone
Anatomic Moment The Temporal Bone Joel D. Swartz, David L. Daniels, H. Ric Harnsberger, Katherine A. Shaffer, and Leighton Mark Despite the advent of thin-section high-reso- the inner ear structures and the external lution computed tomography and, more re- environment. cently, unique magnetic resonance imaging se- Virtually all textbooks define the inner ear as quences with thin sections, T2 weighting, and a membranous labyrinth housed within an os- maximum-intensity projection techniques, seous labyrinth. The membranous labyrinth three-dimensional neuroanatomy of the tempo- contains endolymph, a fluid rich in potassium ral bone and related structures remains some- and low in sodium, similar to intracellular fluid. what of an enigma to many medical specialists, Interposed between the membranous labyrinth neuroradiologists included. Therefore, we are and the osseous labyrinth resides a supportive undertaking a series of anatomic moments in perilymphatic labyrinth. Perilymph is similar to the hope of solidifying the most important ana- cerebrospinal fluid and other extracellular tissue tomic concepts as they relate to this region. Our fluid. approach will be organized so as to consider the The osseous labyrinth consists of the bony temporal bone to represent the complex inter- edifice for the vestibule, semicircular canals, relationship of three systems: hearing, balance, cochlea, and vestibular aqueduct. The mem- and related neuroanatomic and neurovascular branous labyrinth consists of the utricle and structures. saccule (located within the vestibule), the semi- The ear originated in fish as a water motion circular ducts, the cochlear duct, and the en- detection system (1). The vestibular (balance) dolymphatic duct. The latter is a channel within mechanism becomes more complex as we the vestibular aqueduct with communications to scale the embryologic ladder and endolymph the utricle and saccule. -

The Development of the Inner Ear of the Lizard (Sceloporus Undulatus)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Illinois Digital Environment for Access to Learning and Scholarship Repository Digitized by the Internet Archive in 2013 http://archive.org/details/developmentofinnOOgour THE DEVELOPMENT OF THE INNER EAR OF THE LIZARD, (SCELOPORUS UNDULATUS) BY MARY JANE GOURLEY A. B. University of Illinois, 1909. THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF ARTS IN ZOOLOGY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS^ 1914 UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL 190 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE Recommendation concurred in: Committee i on Final Examination II 284573 UlUC TABLE OF CONTENTS Page I Introduction _______ i Methods and Materials II Development of the Inner Ear of the Lizard Stage I _______ 1 Stage II - __-__ - 2 Stage III _______ 3 Stage IV------ - 4 Stage V _______ 5 Stage VI------ - 7 Stage VII------ - 8 Stage VIII _______ 8 III SuMfiary ________ 9 Literature 10 _______ Explanation of Plates - • - - - - 12 Plates ________ 13 . 1 I INTRODUCTION The following paper describes the embryonic development of the inner ear and especially the membranous labyrinth of the lizard ( Soel oporus undulatus ) . Saggital and transverse serial sections of various stages in the development of these animals were studied and wax reconstructions were made of the important stages. Then the sensory area of each section was outlined and painted on the model, so that they shov/ed the limits of the sensory and non-sensory epi- thelium. -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -

The Nervous System: General and Special Senses
18 The Nervous System: General and Special Senses PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Sensory information arrives at the CNS • Information is “picked up” by sensory receptors • Sensory receptors are the interface between the nervous system and the internal and external environment • General senses • Refers to temperature, pain, touch, pressure, vibration, and proprioception • Special senses • Refers to smell, taste, balance, hearing, and vision © 2012 Pearson Education, Inc. Receptors • Receptors and Receptive Fields • Free nerve endings are the simplest receptors • These respond to a variety of stimuli • Receptors of the retina (for example) are very specific and only respond to light • Receptive fields • Large receptive fields have receptors spread far apart, which makes it difficult to localize a stimulus • Small receptive fields have receptors close together, which makes it easy to localize a stimulus. © 2012 Pearson Education, Inc. Figure 18.1 Receptors and Receptive Fields Receptive Receptive field 1 field 2 Receptive fields © 2012 Pearson Education, Inc. Receptors • Interpretation of Sensory Information • Information is relayed from the receptor to a specific neuron in the CNS • The connection between a receptor and a neuron is called a labeled line • Each labeled line transmits its own specific sensation © 2012 Pearson Education, Inc. Interpretation of Sensory Information • Classification of Receptors • Tonic receptors