Vestibular Neuritis, Labyrinthitis, and a Few Comments Regarding Sudden Sensorineural Hearing Loss Marcello Cherchi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
HEARING LOSS, DEAFNESS Ear32 (1)
HEARING LOSS, DEAFNESS Ear32 (1) Hearing Loss, Deafness Last updated: May 11, 2019 CLASSIFICATION, DIAGNOSIS ................................................................................................................. 1 METHODS OF COMMUNICATION FOR DEAF ............................................................................................ 2 MANAGEMENT ......................................................................................................................................... 2 HEARING AIDS (S. AMPLIFICATION) ....................................................................................................... 2 COCHLEAR IMPLANTS ............................................................................................................................ 2 AUDITORY BRAINSTEM IMPLANTS .......................................................................................................... 4 PREVENTION .......................................................................................................................................... 4 PEDIATRIC HEARING DEFICITS ............................................................................................................... 4 ETIOLOGY .............................................................................................................................................. 4 DIAGNOSIS ............................................................................................................................................. 6 TREATMENT .......................................................................................................................................... -

Pediatric Sensorineural Hearing Loss, Part 2: Syndromic And
Published May 19, 2011 as 10.3174/ajnr.A2499 Pediatric Sensorineural Hearing Loss, Part 2: REVIEW ARTICLE Syndromic and Acquired Causes B.Y. Huang SUMMARY: This article is the second in a 2-part series reviewing neuroimaging in childhood SNHL. C. Zdanski Previously, we discussed the clinical work-up of children with hearing impairment, the classification of inner ear malformations, and congenital nonsyndromic causes of hearing loss. Here, we review and M. Castillo illustrate the most common syndromic hereditary and acquired causes of childhood SNHL, with an emphasis on entities that demonstrate inner ear abnormalities on cross-sectional imaging. Syndromes discussed include BOR syndrome, CHARGE syndrome, Pendred syndrome, Waardenburg syndrome, and X-linked hearing loss with stapes gusher. We conclude the article with a review of acquired causes of childhood SNHL, including infections, trauma, and neoplasms. ABBREVIATIONS: BOR ϭ branchio-oto-renal; CISS ϭ constructive interference in steady state; IAC ϭ internal auditory canal; NF-2 ϭ neurofibromatosis type II; SCC ϭ semicircular canal; SNHL ϭ sensorineural hearing loss; T1WI ϭ T1-weighted image; T2 WI ϭ T2-weighted image he estimated prevalence of SNHL in patients younger than Table 1: Selected hereditary syndromes commonly associated with 1 T18 years of age is 6 per 1000, making it one of the leading SNHL causes of childhood disability and a common reason for oto- Inner Ear Malformations on Inner Ear Malformations laryngology referrals. Cross-sectional imaging is now rou- Imaging Not Common on Imaging tinely performed in these patients because it provides impor- Alagille syndrome Alport syndrome tant information about potential etiologies for hearing loss, Branchio-oto-renal syndrome Biotinidase deficiency defines the anatomy of the temporal bone and the central au- CHARGE syndrome Jervell and Lange-Nielsen syndrome ditory pathway, and identifies additional intracranial abnor- Klippel-Feil syndrome Norrie syndrome malities that may require further work-up. -

PDF in English
Case Report Article Labyrinthitis Ossificans. Report of One Case and Literature Review Leandro Ricardo Mattiola*, Mark Makowiecky**, Carlos Eduardo Guimarães de Salles**, Marcela Pozzi Cardoso***, Samir Cahali****. * ENT doctor. Post-graduation student in Head and Neck Surgery at HSPE-SP. ** 2nd yr. Resident Doctor in ENT and Head and Neck Surgery at HSPE-SP. *** ENT Doctor. Assistant doctor in the Otology Department at HSPE-SP. **** PhD in Otorhinolaryngology by UNIFESP. Head of ENT and Head and Neck Surgery Department at HSPE-SP. Institution: Hospital do Servidor Público Estadual de São Paulo - FMO. Departamento de Otorrinolaringologia e Cirurgia de Cabeça e Pescoço. São Paulo / SP – Brazil. Address for correspondence: Leandro Ricardo Mattiola – Rua José de Magalhães 600 - Vila Clementino – São Paulo / SP – Brazil - Zip code: 04026-090 – Telephone/ Fax: (+55 11) 5088-8406 - E-mail: [email protected] Article received on May 31st, 2007. Article approved on November 8th, 2007. SUMMARY Introduction: Labyrinthitis ossificans is a pathology characterized by sensorioneural hearing loss; secondary to infectious process, which produces irreversible injury to inner ear. Objective: To report a labyrinthitis ossificans case and review the literature. Case Report: A seven-year-old male patient, with profound hearing loss in tonal audiometry; no response from brainstem audiometry and compatible CT findings. Conclusion: Labyrinthitis ossificans results ossification on the inner ear structures. Pacient presents profound irreversible hearing loss, followed or not by disequilibrium, that can have important implication on educational socio-development. Diagnosis is important for cochlear implantation cases of the selected cases. Key words: labirinthitis, cochlea, hearing loss, osteogenesis 300 Intl. Arch. Otorhinolaryngol., São Paulo, v.12, n.2, p. -

CASE REPORT 48-Year-Old Man
THE PATIENT CASE REPORT 48-year-old man SIGNS & SYMPTOMS – Acute hearing loss, tinnitus, and fullness in the left ear Dennerd Ovando, MD; J. Walter Kutz, MD; Weber test lateralized to the – Sergio Huerta, MD right ear Department of Surgery (Drs. Ovando and Huerta) – Positive Rinne test and and Department of normal tympanometry Otolaryngology (Dr. Kutz), UT Southwestern Medical Center, Dallas; VA North Texas Health Care System, Dallas (Dr. Huerta) Sergio.Huerta@ THE CASE UTSouthwestern.edu The authors reported no A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of potential conflict of interest hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, relevant to this article. otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications. The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateral- ized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal. THE DIAGNOSIS Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineu- ral and not conductive, ruling out a middle ear effusion. -

Hearing Screening Training Manual REVISED 12/2018
Hearing Screening Training Manual REVISED 12/2018 Minnesota Department of Health (MDH) Community and Family Health Division Maternal and Child Health Section 1 2 For more information, contact Minnesota Department of Health Maternal Child Health Section 85 E 7th Place St. Paul, MN 55164-0882 651-201-3760 [email protected] www.health.state.mn.us Upon request, this material will be made available in an alternative format such as large print, Braille or audio recording. 3 Revisions made to this manual are based on: Guidelines for Hearing Screening After the Newborn Period to Kindergarten Age http://www.improveehdi.org/mn/library/files/afternewbornperiodguidelines.pdf American Academy of Audiology, Childhood Screening Guidelines http://www.cdc.gov/ncbddd/hearingloss/documents/AAA_Childhood%20Hearing%2 0Guidelines_2011.pdf American Academy of Pediatrics (AAP), Hearing Assessment in Children: Recommendations Beyond Neonatal Screening http://pediatrics.aappublications.org/content/124/4/1252 4 Contents Introduction .................................................................................................................... 7 Audience ..................................................................................................................... 7 Purpose ....................................................................................................................... 7 Overview of hearing and hearing loss ............................................................................ 9 Sound, hearing, and hearing -

Migraine Associated Vertigo
Headache: The Journal of Head and Face Pain VC 2015 American Headache Society Published by JohnWiley & Sons, Inc. doi: 10.1111/head.12704 Headache Toolbox Migraine Associated Vertigo Between 30 and 50% of migraineurs will sometimes times a condition similar to benign positional vertigo experience dizziness, a sense of spinning, or feeling like called vestibular neuronitis (or vestibular neuritis/labyrinthi- their balance is off in the midst of their headaches. This is tis) is triggered by a viral infection of the inner ear, result- now termed vestibular migraine, but is also called ing in constant vertigo or unsteadiness. Symptoms can migraine associated vertigo. Sometimes migraineurs last for a few days to a few weeks and then go away as experience these symptoms before the headache, but mysteriously as they came on. Vestibular migraine, by they can occur during the headache, or even without any definition, should have migraine symptoms in at least head pain. In children, vertigo may be a precursor to 50% of the vertigo episodes, and these include head migraines developing in the teens or adulthood. Migraine pain, light and noise sensitivity, and nausea. associated vertigo may be more common in those with There are red flags, which are warning signs that ver- motion sickness. tigo is not part of a migraine. Sudden hearing loss can be For some patients this vertiginous sensation resem- the sign of an infection that needs treatment. Loss of bal- bles migraine aura, which is a reversible, relatively short- ance alone, or accompanied by weakness can be the lived neurologic symptom associated with their migraines. -

Common Vestibular Function Tests
Common Vestibular Function Tests Authors: Barbara Susan Robinson, PT, DPT; Lisa Heusel-Gillig PT DPT NCS Fact Sheet The purpose of Vestibular Function Tests (VFTs) is to determine the health of the vestibular portion of the inner ear. These tests are commonly performed by ENTs, Audiologists, and Otolaryngologists Electronystagmography or Videonystagmography Electronystagmography (ENG test) or Videonystagmography (VNG test) evaluate the inner ear. Both record eye movements during a group of tests in light and dark rooms. During the ENG test, small electrodes are placed on the skin near the eyes to record eye movements. For the VNG test, eye movements are recorded by a video camera mounted inside of goggles that are worn during testing. ENG and VNG tests evaluate eye movements while following a visual target (tracking Produced by test) or during body and head position changes (positional test). The caloric test evaluates eye movements in response to cool or warm air (or water) placed in the ear canal. If there is no response to warm or cool air or water, ice water may be used in order to try to produce a response. The caloric test determines the difference between the function of the left and right inner ear. During this test, you may experience dizziness or nausea. You may be asked questions (math questions, city names, alphabet tasks) to distract you in order to get the best results. A Special Interest Group of Contact us: ANPT Other Common Vestibular Function Tests 5841 Cedar Lake Rd S. The rotary chair test is used along with the VNG to confirm the diagnosis and assess Ste 204 compensation of the vestibular system. -
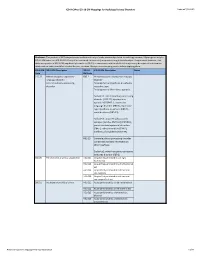
ICD-9/10 Mapping Spreadsheet
ICD-9-CM to ICD-10-CM Mappings for Audiology Related Disorders Updated 7/16/2015 Disclaimer: This product is NOT comprehensive and consists only of codes commonly related to audiology services. Mappings are only to ICD-10-CM codes, not ICD-10-PCS. Every effort was made to accurately map codes using detailed analysis. Keep in mind, however, that while many codes in ICD-9-CM map directly to codes in ICD-10, in some cases, additional clinical analysis may be required to determine which code or codes should be selected for your situation. Always review mapping results before applying them. ICD-9-CM ICD-9-CM Description ICD-10- ICD-10-CM Description Notes Code CM Code 315.32 Mixed receptive-expressive F80.2 Mixed receptive-expressive language language disorder disorder Central auditory processing Developmental dysphasia or aphasia, disorder receptive type Developmental Wernicke's aphasia Excludes1: central auditory processing disorder (H93.25), dysphasia or aphasia NOS (R47.-), expressive language disorder (F80.1), expressive type dysphasia or aphasia (F80.1), word deafness (H93.25) Excludes2: acquired aphasia with epilepsy [Landau-Kleffner] (G40.80-), pervasive developmental disorders (F84.-), selective mutism (F94.0), intellectual disabilities (F70-F79) H93.25 Central auditory processing disorder Congenital auditory imperception Word deafness Excludes1: mixed receptive-epxressive language disorder (F80.2) 380.00 Perichondritis of pinna, unspecified H61.001 Unspecified perichondritis of right external ear H61.002 Unspecified perichondritis -

Vestibular Neuritis and Labyrinthitis
Vestibular Neuritis and DISORDERS Labyrinthitis: Infections of the Inner Ear By Charlotte L. Shupert, PhD with contributions from Bridget Kulick, PT and the Vestibular Disorders Association INFECTIONS Result in damage to inner ear and/or nerve. ARTICLE 079 DID THIS ARTICLE HELP YOU? SUPPORT VEDA @ VESTIBULAR.ORG Vestibular neuritis and labyrinthitis are disorders resulting from an 5018 NE 15th Ave. infection that inflames the inner ear or the nerves connecting the inner Portland, OR 97211 ear to the brain. This inflammation disrupts the transmission of sensory 1-800-837-8428 information from the ear to the brain. Vertigo, dizziness, and difficulties [email protected] with balance, vision, or hearing may result. vestibular.org Infections of the inner ear are usually viral; less commonly, the cause is bacterial. Such inner ear infections are not the same as middle ear infections, which are the type of bacterial infections common in childhood affecting the area around the eardrum. VESTIBULAR.ORG :: 079 / DISORDERS 1 INNER EAR STRUCTURE AND FUNCTION The inner ear consists of a system of fluid-filled DEFINITIONS tubes and sacs called the labyrinth. The labyrinth serves two functions: hearing and balance. Neuritis Inflamation of the nerve. The hearing function involves the cochlea, a snail- shaped tube filled with fluid and sensitive nerve Labyrinthitis Inflamation of the labyrinth. endings that transmit sound signals to the brain. Bacterial infection where The balance function involves the vestibular bacteria infect the middle organs. Fluid and hair cells in the three loop-shaped ear or the bone surrounding semicircular canals and the sac-shaped utricle and Serous the inner ear produce toxins saccule provide the brain with information about Labyrinthitis that invade the inner ear via head movement. -

Diseases of the Brainstem and Cranial Nerves of the Horse: Relevant Examination Techniques and Illustrative Video Segments
IN-DEPTH: NEUROLOGY Diseases of the Brainstem and Cranial Nerves of the Horse: Relevant Examination Techniques and Illustrative Video Segments Robert J. MacKay, BVSc (Dist), PhD, Diplomate ACVIM Author’s address: Alec P. and Louise H. Courtelis Equine Teaching Hospital, College of Veterinary Medicine, University of Florida, Gainesville, FL 32610; e-mail: mackayr@ufl.edu. © 2011 AAEP. 1. Introduction (pons and cerebellum) and myelencephalon (me- This lecture focuses on the functions of the portions dulla oblongata). Because the diencephalon was of the brainstem caudal to the diencephalon. In discussed in the previous lecture under Forebrain addition to regulation of many of the homeostatic Diseases, it will not be covered here. mechanisms of the body, this part of the brainstem controls consciousness, pupillary diameter, eye 3. Functions (Location) movement, facial expression, balance, prehension, mastication and swallowing of food, and movement Pupillary Light Response, Pupil Size (Midbrain, Cranial and coordination of the trunk and limbs. Dysfunc- Nerves II, III) tion of the brainstem and/or cranial nerves therefore In the normal horse, pupil size reflects the balance of manifests in a great variety of ways including re- sympathetic (dilator) and parasympathetic (con- duced consciousness, ataxia, limb weakness, dys- strictor) influences on the smooth muscle of the phagia, facial paralysis, jaw weakness, nystagmus, iris.2–4 Preganglionic neurons for sympathetic and strabismus. Careful neurologic examination supply to the head arise in the gray matter of the in the field can provide accurate localization of first four thoracic segments of the spinal cord and brainstem and cranial nerve lesions. Recognition subsequently course rostrally in the cervical sympa- of brainstem/cranial nerve dysfunction is an impor- thetic nerve within the vagosympathetic trunk. -

Inner Ear Infection (Otitis Interna) in Dogs
Hurricane Harvey Client Education Kit Inner Ear Infection (Otitis Interna) in Dogs My dog has just been diagnosed with an inner ear infection. What is this? Inflammation of the inner ear is called otitis interna, and it is most often caused by an infection. The infectious agent is most commonly bacterial, although yeast and fungus can also be implicated in an inner ear infection. If your dog has ear mites in the external ear canal, this can ultimately cause a problem in the inner ear and pose a greater risk for a bacterial infection. Similarly, inner ear infections may develop if disease exists in one ear canal or when a benign polyp is growing from the middle ear. A foreign object, such as grass seed, may also set the stage for bacterial infection in the inner ear. Are some dogs more susceptible to inner ear infection? Dogs with long, heavy ears seem to be predisposed to chronic ear infections that ultimately lead to otitis interna. Spaniel breeds, such as the Cocker spaniel, and hound breeds, such as the bloodhound and basset hound, are the most commonly affected breeds. Regardless of breed, any dog with a chronic ear infection that is difficult to control may develop otitis interna if the eardrum (tympanic membrane) is damaged as it allows bacteria to migrate down into the inner ear. "Dogs with long, heavy ears seem to bepredisposed to chronic ear infections that ultimately lead to otitis interna." Excessively vigorous cleaning of an infected external ear canal can sometimes cause otitis interna. Some ear cleansers are irritating to the middle and inner ear and can cause signs of otitis interna if the eardrum is damaged and allows some of the solution to penetrate too deeply. -

Hearing Loss, Vertigo and Tinnitus
HEARING LOSS, VERTIGO AND TINNITUS Jonathan Lara, DO April 29, 2012 Hearing Loss Facts S Men are more likely to experience hearing loss than women. S Approximately 17 percent (36 million) of American adults report some degree of hearing loss. S About 2 to 3 out of every 1,000 children in the United States are born deaf or hard-of-hearing. S Nine out of every 10 children who are born deaf are born to parents who can hear. Hearing Loss Facts S The NIDCD estimates that approximately 15 percent (26 million) of Americans between the ages of 20 and 69 have high frequency hearing loss due to exposure to loud sounds or noise at work or in leisure activities. S Only 1 out of 5 people who could benefit from a hearing aid actually wears one. S Three out of 4 children experience ear infection (otitis media) by the time they are 3 years old. Hearing Loss Facts S There is a strong relationship between age and reported hearing loss: 18 percent of American adults 45-64 years old, 30 percent of adults 65-74 years old, and 47 percent of adults 75 years old or older have a hearing impairment. S Roughly 25 million Americans have experienced tinnitus. S Approximately 4,000 new cases of sudden deafness occur each year in the United States. Hearing Loss Facts S Approximately 615,000 individuals have been diagnosed with Ménière's disease in the United States. Another 45,500 are newly diagnosed each year. S One out of every 100,000 individuals per year develops an acoustic neurinoma (vestibular schwannoma).