The Influence of Cochlear Shape on Low-Frequency Hearing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sound and the Ear Chapter 2
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter© Jones & Bartlett 2 Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Sound and the Ear © Jones Karen &J. Kushla,Bartlett ScD, Learning, CCC-A, FAAA LLC © Jones & Bartlett Learning, LLC Lecturer NOT School FOR of SALE Communication OR DISTRIBUTION Disorders and Deafness NOT FOR SALE OR DISTRIBUTION Kean University © Jones & Bartlett Key Learning, Terms LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR Acceleration DISTRIBUTION Incus NOT FOR SALE OR Saccule DISTRIBUTION Acoustics Inertia Scala media Auditory labyrinth Inner hair cells Scala tympani Basilar membrane Linear scale Scala vestibuli Bel Logarithmic scale Semicircular canals Boyle’s law Malleus Sensorineural hearing loss Broca’s area © Jones & Bartlett Mass Learning, LLC Simple harmonic© Jones motion (SHM) & Bartlett Learning, LLC Brownian motion Membranous labyrinth Sound Cochlea NOT FOR SALE OR Mixed DISTRIBUTION hearing loss Stapedius muscleNOT FOR SALE OR DISTRIBUTION Compression Organ of Corti Stapes Condensation Osseous labyrinth Tectorial membrane Conductive hearing loss Ossicular chain Tensor tympani muscle Decibel (dB) Ossicles Tonotopic organization © Jones Decibel & hearing Bartlett level (dB Learning, HL) LLC Outer ear © Jones Transducer & Bartlett Learning, LLC Decibel sensation level (dB SL) Outer hair cells Traveling wave theory NOT Decibel FOR sound SALE pressure OR level DISTRIBUTION -

The Standing Acoustic Wave Principle Within the Frequency Analysis Of
inee Eng ring al & ic d M e e d Misun, J Biomed Eng Med Devic 2016, 1:3 m i o c i a B l D f o e v DOI: 10.4172/2475-7586.1000116 l i a c n e r s u o Journal of Biomedical Engineering and Medical Devices J ISSN: 2475-7586 Review Article Open Access The Standing Acoustic Wave Principle within the Frequency Analysis of Acoustic Signals in the Cochlea Vojtech Misun* Department of Solid Mechanics, Mechatronics and Biomechanics, Brno University of Technology, Brno, Czech Republic Abstract The organ of hearing is responsible for the correct frequency analysis of auditory perceptions coming from the outer environment. The article deals with the principles of the analysis of auditory perceptions in the cochlea only, i.e., from the overall signal leaving the oval window to its decomposition realized by the basilar membrane. The paper presents two different methods with the function of the cochlea considered as a frequency analyzer of perceived acoustic signals. First, there is an analysis of the principle that cochlear function involves acoustic waves travelling along the basilar membrane; this concept is one that prevails in the contemporary specialist literature. Then, a new principle with the working name “the principle of standing acoustic waves in the common cavity of the scala vestibuli and scala tympani” is presented and defined in depth. According to this principle, individual structural modes of the basilar membrane are excited by continuous standing waves of acoustic pressure in the scale tympani. Keywords: Cochlea function; Acoustic signals; Frequency analysis; The following is a description of the theories in question: Travelling wave principle; Standing wave principle 1. -

Low-Frequency Noise: a Biophysical Phenomenon M
PSC REF#:288480 Public Service Commission of Wisconsin RECEIVED: 07/08/16, 8:46:12 AM Congres Geluid, Trillingen, Luchtkwaliteit en Gebied & Gebouw 2012 Low-frequency noise: a biophysical phenomenon M. Oud (medical physicist / consultant)* * [email protected], http://nl.linkedin.com/in/mireilleoud, the Netherlands Abstract Complaints on low-frequency noise were till recently fairly unexplained, but audiological research shed light on the mechanisms that enable perception of frequencies below the threshold of average normal hearing. It was shown that exposure to low-frequency sound may alter the inner ear. This results in an increase of sensitivity to low-frequency sounds, and as a result, previously imperceptible sounds becomes audible to the exposed person. Interactions between inner-ear responses to low and higher frequencies furthermore account for perception of low-frequency sound, as well as the property of the hearing system to perceive so-called difference tones. Introduction A growing minority of people experiences an increased sensitivity for low-frequency sound. Not surprisingly, they complain about noise, even about loud noise in some cases. Their complaints about the presence of hum, buzz, and rumble are often not recognized as a nuisance, since the majority of people does not perceive the very low frequencies. Low-frequency noise (LFN) may have serious health effects like vertigo, disturbed sleep, stress, hypertension, and heart rhythm disorders [1]. The number of sufferers is growing, and this has two possible causes. The sources of low- frequency sounds increased in volume and dimension over the past decades, and auditory sensitisation takes years to develop. Nowadays, the main source of low-frequency noise is the public infrastructure: wind turbines, gas transmission grid, industrial plants, road and railway traffic, sewerage, and so on. -

CONGENITAL MALFORMATIONS of the INNER EAR Malformaciones Congénitas Del Oído Interno
topic review CONGENITAL MALFORMATIONS OF THE INNER EAR Malformaciones congénitas del oído interno. Revisión de tema Laura Vanessa Ramírez Pedroza1 Hernán Darío Cano Riaño2 Federico Guillermo Lubinus Badillo2 Summary Key words (MeSH) There are a great variety of congenital malformations that can affect the inner ear, Ear with a diversity of physiopathologies, involved altered structures and age of symptom Ear, inner onset. Therefore, it is important to know and identify these alterations opportunely Hearing loss Vestibule, labyrinth to lower the risks of all the complications, being of great importance, among others, Cochlea the alterations in language development and social interactions. Magnetic resonance imaging Resumen Existe una gran variedad de malformaciones congénitas que pueden afectar al Palabras clave (DeCS) oído interno, con distintas fisiopatologías, diferentes estructuras alteradas y edad Oído de aparición de los síntomas. Por lo anterior, es necesario conocer e identificar Oído interno dichas alteraciones, con el fin de actuar oportunamente y reducir el riesgo de las Pérdida auditiva Vestíbulo del laberinto complicaciones, entre otras —de gran importancia— las alteraciones en el área del Cóclea lenguaje y en el ámbito social. Imagen por resonancia magnética 1. Epidemiology • Hyperbilirubinemia Ear malformations occur in 1 in 10,000 or 20,000 • Respiratory distress from meconium aspiration cases (1). One in every 1,000 children has some degree • Craniofacial alterations (3) of sensorineural hearing impairment, with an average • Mechanical ventilation for more than five days age at diagnosis of 4.9 years. The prevalence of hearing • TORCH Syndrome (4) impairment in newborns with risk factors has been determined to be 9.52% (2). -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

The Special Senses the Ear External Ear Middle
1/24/2016 The Ear • The organ of hearing and equilibrium – Cranial nerve VIII - Vestibulocochlear – Regions The Special Senses • External ear • Middle ear Hearing and • Internal ear (labyrinth) Equilibrium External Ear Middle Internal ear • Two parts External ear (labyrinth) ear – Pinna or auricle (external structures) – External auditory meatus (car canal) Auricle • Site of cerumen (earwax) production (pinna) – Waterproofing, protection • Separated from the middle ear by the tympanic membrane Helix (eardrum) – Vibrates in response to sound waves Lobule External acoustic Tympanic Pharyngotympanic meatus membrane (auditory) tube (a) The three regions of the ear Figure 15.25a Middle Ear Epitympanic Middle Ear Superior Malleus Incus recess Lateral • Tympanic cavity Anterior – Air-filled chamber – Openings View • Tympanic membrane – covers opening to outer ear • Round and oval windows – openings to inner ear • Epitympanic recess – dead-end cavity into temporal bone of unknown function • Auditory tube – AKA Eustachian tube or pharyngotympanic tube Pharyngotym- panic tube Tensor Tympanic Stapes Stapedius tympani membrane muscle muscle (medial view) Figure 15.26 1 1/24/2016 Middle Ear Middle Ear • Auditory tube (Eustachian tube) • Otitis Media – Connects the middle ear to the nasopharynx • Equalizes pressure – Opens during swallowing and yawning Middle Ear Middle Ear • Contains auditory ossicles (bones) • Sound waves cause tympanic membrane to vibrate – Malleus • Ossicles help transmit vibrations into the inner ear – Incus – Reduce the area -

Turbulence Around the Otoliths Sappey's Hostility and Br
B-ENT, 2006, 2, 99-102 A Historical Vignette “Be proud of yourself: you have a History!” Turbulence around the otoliths Sappey’s hostility and Breschet’s defence J. Tainmont W. Churchill Avenue 172, 1180 Brussels, Belgium Key-words. Vestibular apparatus; otoliths; Breschet; Sappey; history of otology Abstract. Turbulence around the otoliths. Sappey’s hostility and Breschet’s defence. Nowadays, the animosity between medical scholars is seldom apparent. However, during the XIXth century it was not necessarily so. We find an example of this in Sappey’s hostility against his colleague anatomist Gilbert Breschet. It concerned the discovery of the otoliths of the inner ear that Breschet attributed to himself. We present here Breschet’s defence. Sappey’s hostility (Figure 1) Marie-Philibert-Constant Sappey was a French anatomist who received from the 1900 Larousse Encyclopaedia the honour of an article with a pen-and-ink draw- ing. He was born at Bourg (Ain department) in 1810 and he died in Paris in 1896. He published important works concerning the lymphatic vessels. He described their anatomy, physiology, pathol- ogy and iconography. We owe him the knowledge of the lymphatic vessels and nodes of the supra- glottic part of the larynx (1889) AB that was the basis of the radical neck dissection for laryngeal can- Figure 1 cer. Nevertheless, Sappey was also A. Sappey (1810-1896). B. Lymphatic vessels of head and neck with the great lymphatic vein1. an irascible man! In his treatise on descriptive anatomy (1845-1863), in the chapter “sense of hearing”, we find a history of the discovery “After the work of Scarpa, was very simple, to mention it was of the membranous labyrinth into Breschet’s one appeared… In that enough. -

The Special Senses
HOMEWORK DUE IN LAB 5 HW page 9: Matching Eye Disorders PreLab 5 THE SPECIAL SENSES Hearing and Equilibrium THE EAR The organ of hearing and equilibrium . Cranial nerve VIII - Vestibulocochlear . Regions . External ear . Middle ear . Internal ear (labyrinth) Middle Internal ear External ear (labyrinth) ear Auricle (pinna) Helix Lobule External acoustic Tympanic Pharyngotympanic meatus membrane (auditory) tube (a) The three regions of the ear Figure 15.25a Middle Ear Epitympanic Superior Malleus Incus recess Lateral Anterior View Pharyngotym- panic tube Tensor Tympanic Stapes Stapedius tympani membrane muscle muscle (medial view) Copyright © 2010 Pearson Education, Inc. Figure 15.26 MIDDLE EAR Auditory tube . Connects the middle ear to the nasopharynx . Equalizes pressure . Opens during swallowing and yawning . Otitis media INNER EAR Contains functional organs for hearing & equilibrium . Bony labyrinth . Membranous labyrinth Superior vestibular ganglion Inferior vestibular ganglion Temporal bone Semicircular ducts in Facial nerve semicircular canals Vestibular nerve Anterior Posterior Lateral Cochlear Cristae ampullares nerve in the membranous Maculae ampullae Spiral organ Utricle in (of Corti) vestibule Cochlear duct Saccule in in cochlea vestibule Stapes in Round oval window window Figure 15.27 INNER EAR - BONY LABYRINTH Three distinct regions . Vestibule . Gravity . Head position . Linear acceleration and deceleration . Semicircular canals . Angular acceleration and deceleration . Cochlea . Vibration Superior vestibular ganglion Inferior vestibular ganglion Temporal bone Semicircular ducts in Facial nerve semicircular canals Vestibular nerve Anterior Posterior Lateral Cochlear Cristae ampullares nerve in the membranous Maculae ampullae Spiral organ Utricle in (of Corti) vestibule Cochlear duct Saccule in in cochlea vestibule Stapes in Round oval window window Figure 15.27 INNER EAR The cochlea . -

Tympanic Membrane (Membrana Tympanica, Myrinx)
Auditory and vestibular system Auris, is = Us, oton Auditory and vestibular system • external ear (auris externa) • middle ear (auris media) • internal ear (auris interna) = organum vestibulo- cochleare External ear (Auris externa) • auricle (auricula, pinna) – elastic cartilage • external acoustic meatus (meatus acusticus externus) • tympanic membrane (membrana tympanica, myrinx) • helix Auricle – crus, spina, cauda – (tuberculum auriculare Darwini, apex auriculae) • antihelix – crura, fossa triangularis • scapha • concha auriculae – cymba, cavitas • tragus • antitragus • incisura intertragica • lobulus auriculae posterior surface = negative image of the anterior one ligaments: lig. auriculare ant., sup., post. muscles – innervation: n. facialis • extrinsic muscles = facial muscles – mm. auriculares (ant., sup., post.) – m. temporoparietalis • intrinsic muscles: rudimentary – m. tragicus + antitragicus – m. helicis major+minor – m. obliquus + transversus auriculae, m. pyramidalis auriculae cartilage: cartilago auriculae - elastic skin: dorsally more loosen, ventrally firmly fixed to perichondrium - othematoma Auricle – supply • arteries: a. temporalis superficialis → rr. auriculares ant. a. carotis externa → a. auricularis post. • veins: v. jugularis ext. • lymph: nn.ll. parotidei, mastoidei • nerves: sensory – nn. auriculares ant. from n. auriculotemporalis (ventrocranial 2/3) – r. auricularis n. X. (concha) – n. occipitalis minor (dosrocranial) – n. auricularis magnus (cudal) motor: n. VII. External acoustic meatus (meatus acusticus -

16.400 Human Factors Engineering, Lecture 6 Notes
Spatial Disorientation 16.400/16.453 Human Factors Engineering Prof. L. Young Sept. 2011 1 SPATIAL DISORIENTATION IN FLIGHT Formal Definition “[A failure] to sense correctly the position, motion or attitude of his aircraft or of himself [herself] within the fixed coordinate system provided by the surface of the earth and the gravitational vertical. In addition, errors in perception by the aviator of his position, motion or attitude with respect to his aircraft, or of his own aircraft relative to other aircraft, may also be embraced within a broader definition of spatial disorientation in flight. -- Alan Benson (1978) 2 SPATIAL DISORIENTATION IN FLIGHT Spatial Disorientation Types TYPE I -- Unrecognized • Pilot Does Not Consciously Perceive Any Manifestation of Spatial Disorientation • Most Often Occurs When Pilot Breaks Instrument Cross-Check • Most Likely to Lead to Controlled Flight Into Terrain 3 SPATIAL DISORIENTATION IN FLIGHT Spatial Disorientation Types TYPE II -- Recognized • Pilot Consciously Perceives A Manifestation of Spatial Disorientation but May Not Attribute It to SD Itself • Conflict between “Natural” and “Synthetic” SD Percepts May Occur • Instrument Malfunction Is Often Suspected 4 SPATIAL DISORIENTATION IN FLIGHT Spatial Disorientation Types TYPE III -- Incapacitating • Experienced by 10-15% of Aviators • Vestibulo-Ocular Disorganization (i.e., uncontrollable nystagmus) • Motor Conflict (e.g., “Giant Hand”) • Temporal Distortion • Dissociation (“Break-Off”) 5 SPATIAL DISORIENTATION IN FLIGHT Predisposing Perceptual -
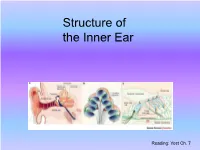
Structure of the Inner Ear
Structure of the Inner Ear Reading: Yost Ch. 7 The Mammalian Ear The Inner Ear Inner ear contains two sensory structures in one organ: • Vestibular apparatus, which contains mechanosensory organs for balance (orientation re gravity), head acceleration in three dimensions. • Cochlea, which contains the mechanosensory epithelium for hearing (“Organ of Corti”). Vestibular and cochlear output fibers gather to become the VIIIth cranial nerve (vestibulocochlear). The Cochlea Transduction: Transmission: • Cochlea biomechanically segregates • Auditory hair cell receptors activate sound frequencies along the basilar auditory nerve fibers (ANFs). membrane • ANFs encode information about the • Basilar membrane creates a “filter frequency, amplitude, and timing of bank” of auditory receptors (“hair the acoustic stimulus, and relay that cells”), which convert sound energy encoded information to the central into neurophysiological responses. auditory system. Human Cochlea A bony, three-chambered tubular Apex structure. Coiled into a 2.5-turn helix, 35 mm long (human) from base (stapes/oval window) to apex. Central axis of helix is refered to as the modiolus (mō-dī´-ō-lus). The cell bodies of spiral ganglion cells (auditory nerve Base fibers) are located in the modiolus. Basilar membrane (BM; “cochlear partition”) extends along entire length Basilar Oval membrane Organ of Corti (sensory epithelium) window rests on the BM. Stapes Round window The Vestibule Oval window membrane attached to stapes, faces into the vestibule. The bony labyrinth is the rigid outer wall of the inner ear. It consists of three parts: the vestibule, semicircular canals and cochlea. They contain a clear fluid (brown), called the perilymph, in which floats the membranous labyrinth (blue).