Determination of the Round Window Niche Anatomy Using Cone Beam Computed Tomography Imaging As Preparatory Work for Individualized Drug-Releasing Implants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Pictorial Review of Round Window Pathologies
REVIEW ARTICLE The Forgotten Second Window: A Pictorial Review of Round Window Pathologies J.C. Benson, F. Diehn, T. Passe, J. Guerin, V.M. Silvera, M.L. Carlson, and J. Lane ABSTRACT SUMMARY: The round window serves to decompress acoustic energy that enters the cochlea via stapes movement against the oval window. Any inward motion of the oval window via stapes vibration leads to outward motion of the round window. Occlusion of the round window is a cause of conductive hearing loss because it increases the resistance to sound energy and con- sequently dampens energy propagation. Because the round window niche is not adequately evaluated by otoscopy and may be incompletely exposed during an operation, otologic surgeons may not always correctly identify associated pathology. Thus, radiol- ogists play an essential role in the identification and classification of diseases affecting the round window. The purpose of this review is to highlight the developmental, acquired, neoplastic, and iatrogenic range of pathologies that can be encountered in round window dysfunction. ABBREVIATIONS: LO 4 labyrinthitis ossificans; RW 4 round window he round window (RW) serves as a boundary between the considerations of the round window that can be encoun- Tbasal turn of the cochlea anteriorly and the round win- tered on imaging are reviewed. dow niche posteriorly.1 It, along with the oval window, is 1 of 2 natural openings between the inner and middle ear. The Physiology and Functionality “ ” round window is often overshadowed by the first window The inner ear “windows” refer to openings in the otic capsule “ ” (the oval window) and pathologic third windows (eg, that connect the fluid in the inner ear to either the middle ear or superior semicircular canal dehiscence). -

The Round Window Region and Contiguous Areas: Endoscopic Anatomy and Surgical Implications
Eur Arch Otorhinolaryngol DOI 10.1007/s00405-014-2923-8 OTOLOGY The round window region and contiguous areas: endoscopic anatomy and surgical implications Daniele Marchioni · Matteo Alicandri-Ciufelli · David D. Pothier · Alessia Rubini · Livio Presutti Received: 16 October 2013 / Accepted: 28 January 2014 © Springer-Verlag Berlin Heidelberg 2014 Abstract The round window region is a critical area of window region. Exact anatomical knowledge of this region the middle ear; the aim of this paper is to describe its anat- can have important advantages during surgery, since some omy from an endoscopic perspective, emphasizing some pathology can invade inside cavities or tunnels otherwise structures, the knowledge of which could have important not seen by instrumentation that produces a straight-line implications during surgery, as well as to evaluate what view (e.g. microscope). involvement cholesteatoma may have with these structures. Retrospective review of video recordings of endoscopic Keywords Retrotympanum · Endoscopic ear surgery · ear surgeries and retrospective database review were con- Cholesteatoma · Middle ear anatomy · Round window ducted in Tertiary university referral center. Videos from endoscopic middle ear procedures carried out between June 2010 and September 2012 and stored in a shared data- Introduction base were reviewed retrospectively. Surgeries in which an endoscopic magnification of the round window region and The surgical management of cholesteatoma is still a contro- the inferior retrotympanum area was performed intraop- versial issue. Endoscopic instrumentation, techniques and eratively were included in the study. Involvement by cho- knowledge have improved considerably over the last few lesteatoma of those regions was also documented based years, and we believe that, in the future, endoscopic surgi- on information obtained from the surgical database. -

Round Window Chamber and Fustis: Endoscopic Anatomy and Surgical Implications
Round window chamber and fustis: endoscopic anatomy and surgical implications Daniele Marchioni, Davide Soloperto, Elena Colleselli, Maria Fatima Tatti, Nirmal Patel & Nicholas Jufas Surgical and Radiologic Anatomy ISSN 0930-1038 Surg Radiol Anat DOI 10.1007/s00276-016-1662-5 1 23 Your article is protected by copyright and all rights are held exclusively by Springer- Verlag France. This e-offprint is for personal use only and shall not be self-archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Surg Radiol Anat DOI 10.1007/s00276-016-1662-5 ORIGINAL ARTICLE Round window chamber and fustis: endoscopic anatomy and surgical implications 1 1 1 1 Daniele Marchioni • Davide Soloperto • Elena Colleselli • Maria Fatima Tatti • 2,3,4 2,3,4 Nirmal Patel • Nicholas Jufas Received: 14 September 2015 / Accepted: 29 February 2016 Ó Springer-Verlag France 2016 Abstract The round window region is of critical which enables a comprehensive exploration of the round importance in the anatomy of the middle ear. The aim of window region. Accurate knowledge of the anatomical this paper is to describe its anatomy from an endoscopic relationships of this region has important advantages point of view, emphasizing structures that have important during middle ear surgery. -
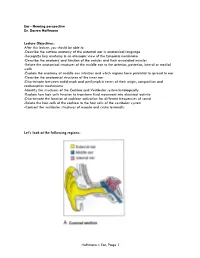
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond
Published August 25, 2016 as 10.3174/ajnr.A4922 REVIEW ARTICLE Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond X M.-L. Ho, X G. Moonis, X C.F. Halpin, and X H.D. Curtin ABSTRACT SUMMARY: Third window abnormalities are defects in the integrity of the bony structure of the inner ear, classically producing sound-/ pressure-induced vertigo (Tullio and Hennebert signs) and/or a low-frequency air-bone gap by audiometry. Specific anatomic defects include semicircular canal dehiscence, perilabyrinthine fistula, enlarged vestibular aqueduct, dehiscence of the scala vestibuli side of the cochlea, X-linked stapes gusher, and bone dyscrasias. We discuss these various entities and provide key examples from our institutional teaching file with a discussion of symptomatology, temporal bone CT, audiometry, and vestibular-evoked myogenic potentials. ABBREVIATIONS: EVAS ϭ enlarged vestibular aqueduct syndrome; SSCCD ϭ superior semicircular canal dehiscence hird window abnormalities are defects in the integrity of the ing effects decrease air conduction. The 2 physiologic windows Tbony structure of the inner ear, first described by Minor et al between the middle and inner ear are the oval window, which in 1998.1 In 2008, Merchant and Rosowski2 proposed a universal transmits vibrations from the auditory ossicles, and the round theory for the underlying mechanism of hearing loss accompany- window of the cochlea. With air conduction, there is physiologic ing these defects. Normal sound conduction is transmitted entrainment of the oval and round windows due to coupling by through the oval and round windows, which serve as fluid inter- incompressible perilymph. Pressure differences between the co- faces between air in the middle ear and perilymphatic fluid spaces chlear perilymphatic spaces activate hair cells and create the per- of the inner ear. -

Measuring Cochlear Duct Length – a Historical Analysis of Methods and Results Robert W
Koch et al. Journal of Otolaryngology - Head and Neck Surgery (2017) 46:19 DOI 10.1186/s40463-017-0194-2 REVIEW Open Access Measuring Cochlear Duct Length – a historical analysis of methods and results Robert W. Koch1*, Hanif M. Ladak1,2,3,4†, Mai Elfarnawany2† and Sumit K. Agrawal1,2,4,5† Abstract Background: Cochlear Duct Length (CDL) has been an important measure for the development and advancement of cochlear implants. Emerging literature has shown CDL can be used in preoperative settings to select the proper sized electrode and develop customized frequency maps. In order to improve post-operative outcomes, and develop new electrode technologies, methods of measuring CDL must be validated to allow usage in the clinic. Purpose: The purpose of this review is to assess the various techniques used to calculate CDL and provide the reader with enough information to make an informed decision on how to conduct future studies measuring the CDL. Results: The methods to measure CDL, the modality used to capture images, and the location of the measurement have all changed as technology evolved. With recent popularity and advancement in computed tomography (CT) imaging in place of histologic sections, measurements of CDL have been focused at the lateral wall (LW) instead of the organ of Corti (OC), due to the inability of CT to view intracochlear structures. After analyzing results from methods such as directly measuring CDL from histology, indirectly reconstructing the shape of the cochlea, and determining CDL based on spiral coefficients, it was determined the three dimensional (3D) reconstruction method is the most reliable method to measure CDL. -

Systematic Review of Round Window Operations for the Treatment of Superior Semicircular Canal Dehiscence
J Int Adv Otol 2019; 15(2): 209-14 • DOI: 10.5152/iao.2019.6550 Review Systematic Review of Round Window Operations for the Treatment of Superior Semicircular Canal Dehiscence Waseem Ahmed , Rajini Rajagopal , Gareth Lloyd Department of Otolaryngology, St. George's Hospital, London, United Kingdom (WA) Department of Otolaryngology, John Radcliffe Hospital, Oxford, United Kingdom (RR) Department of Otolaryngology, Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom (GL) ORCID IDs of the authors: W.A. 0000-0003-3832-1187; R.R. 0000-0003-0453-0845; G.L. 0000-0002-0174-2087. Cite this article as: Ahmed W, Rajagopal R, Lloyd G. Systematic Review of Round Window Operations for the Treatment of Superior Semicircular Canal Dehiscence. J Int Adv Otol 2019; 15(2): 209-14. A review of the literature is presented to consider the role of round window (RW) operations in superior semicircular canal dehiscence (SSCD). Primary (PubMed) and secondary sources (TRIP, Cochrane database, Best Practice, and PubMed Clinical Queries) were used to identify relevant studies. Four original studies (three case series and one case report) were identified. All were retrospective reviews and used a number of subjec- tive and objective outcome measures to assess the efficacy of a minimally invasive, transmeatal approach to perform RW surgery for SSCD. The current evidence suggesting that RW operations for SSCD are unlikely to replace more established surgical procedures as first-line treatment may be appropriate in a select group of patients. Further multicenter, randomized controlled trials are required to establish their efficacy in patients with SSCD. KEYWORDS: Round window, superior semicircular canal dehiscence INTRODUCTION Minor et al. -

Anatomy of the Ear ANATOMY & Glossary of Terms
Anatomy of the Ear ANATOMY & Glossary of Terms By Vestibular Disorders Association HEARING & ANATOMY BALANCE The human inner ear contains two divisions: the hearing (auditory) The human ear contains component—the cochlea, and a balance (vestibular) component—the two components: auditory peripheral vestibular system. Peripheral in this context refers to (cochlea) & balance a system that is outside of the central nervous system (brain and (vestibular). brainstem). The peripheral vestibular system sends information to the brain and brainstem. The vestibular system in each ear consists of a complex series of passageways and chambers within the bony skull. Within these ARTICLE passageways are tubes (semicircular canals), and sacs (a utricle and saccule), filled with a fluid called endolymph. Around the outside of the tubes and sacs is a different fluid called perilymph. Both of these fluids are of precise chemical compositions, and they are different. The mechanism that regulates the amount and composition of these fluids is 04 important to the proper functioning of the inner ear. Each of the semicircular canals is located in a different spatial plane. They are located at right angles to each other and to those in the ear on the opposite side of the head. At the base of each canal is a swelling DID THIS ARTICLE (ampulla) and within each ampulla is a sensory receptor (cupula). HELP YOU? MOVEMENT AND BALANCE SUPPORT VEDA @ VESTIBULAR.ORG With head movement in the plane or angle in which a canal is positioned, the endo-lymphatic fluid within that canal, because of inertia, lags behind. When this fluid lags behind, the sensory receptor within the canal is bent. -

Audiometric Findings with Voluntary Tensor Tympani Contraction Brandon Wickens1 , Duncan Floyd2 and Manohar Bance3*
Wickens et al. Journal of Otolaryngology - Head and Neck Surgery (2017) 46:2 DOI 10.1186/s40463-016-0182-y ORIGINALRESEARCHARTICLE Open Access Audiometric findings with voluntary tensor tympani contraction Brandon Wickens1 , Duncan Floyd2 and Manohar Bance3* Abstract Background: Tensor tympani contraction may have a "signature" audiogram. This study demonstrates audiometric findings during voluntary tensor tympani contraction. Methods: Five volunteers possessing the ability to voluntarily contract their tensor tympani muscles were identified and enrolled. Tensor tympani contraction was confirmed with characteristic tympanometry findings. Study subjects underwent conventional audiometry. Air conduction and bone conduction threshold testing was performed with and without voluntary tensor tympani contraction. Main outcome measure: Changes in air conduction and bone conduction thresholds during voluntary tensor tympani contraction. Results: Audiometric results demonstrate a low frequency mixed hearing loss resulting from tensor tympani contraction. Specifically, at 250 Hz, air conduction thresholds increased by 22 dB and bone conduction thresholds increased by 10 dB. Conclusions: Previous research has demonstrated a low frequency conductive hearing loss in the setting of tensor tympanic contraction. This is the first study to demonstrate a low frequency mixed hearing loss associated with tensor tympani contraction. This finding may aid in the diagnosis of disorders resulting from abnormal tensor tympani function. Tensor tympani contraction -

Anatomic Variations of the Round Window Niche: Radiological Study and Related Endoscopic Anatomy
Surgical and Radiologic Anatomy (2019) 41:853–857 https://doi.org/10.1007/s00276-019-02225-8 ANATOMIC VARIATIONS Anatomic variations of the round window niche: radiological study and related endoscopic anatomy Pietro Canzi1 · Irene Avato1,2 · Marco Manfrin1 · Anna Maria Simoncelli3 · Marianna Magnetto1 · Elisabetta Rebecchi1 · Carmine Tinelli4 · Marinella Neri5 · Millo Achille Beltrame1 · Marco Benazzo1 Received: 2 October 2018 / Accepted: 15 March 2019 / Published online: 21 March 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Purpose In the last decades, literature has shown an increasing interest in round windows (RW) anatomy due to its pivotal role in deafness surgery. The high variability of this anatomical region, with particular regard to the round windows niche (RWN), has been studied by several authors through different methods of investigation. The aim of the present research was to radiologically examine the morphological variability of the RWN and to link the imaging findings to the endoscopic view. Methods High-resolution CT scans of 300 temporal bones without neuro-otological pathologies were retrospectively reviewed by 2 neuroradiologist and 1 ENT surgeon who independently evaluated the RWN morphological variations. To link the radiological to the endoscopic data, 45 cadaveric human temporal bones were submitted to a radiological evaluation and to an otoendoscopy conducted through a posterior tympanotomy approach. Results Three variants of the RWN were detected on coronal CT scan reconstructions: 155 “cylindrical-type”, 97 “j-type” and 48 “truncated cone-type”. For each radiological type the endoscopic findings showed a specific endoscopic position of the RW chamber, which results in different degrees of RW membrane visibility when analysed through a posterior tympa- notomy approach. -

Round Window Electrochleography in Cochlear Implantation
Scholarly Journal of Otolaryngology DOI: 10.32474/SJO.2021.05.000223 ISSN: 2641-1709 Case Report Round Window Electrochleography in Cochlear Implantation Asil Ergin1*, Mehmet Ziya Özüer2 and Levent Olgun3 1ENT Department, Ödemiş State Hospital, Turkey 2ENT Department, Acıbadem University Bodrum Hospital, Turkey 3ENT Department, Başkent University Zübeyde Hanım Hospital, Turkey *Corresponding author: Asil Ergin, ENT Department, Ödemiş State Hospital, İzmir, Turkey Received: Published: January 11, 2021 January 27, 2021 Abstract Introduction: In this study which was achieved at Izmir Bozyaka Teaching and Research Hospital, identifying the sensitivity and reliability of round window electrocochleographic records on children during cochlear implantation and investigating the applicabilityMaterial of and the Methods: method on particularly problematic patient groups was aimed. In this study, the golf-club electrode was placed on the round window niche during cochlear implantation of 30 patients after posterior tympanotomy. Then click stimulus was given by TDH 39 headphones over the speculum which was placed in the external ear canal and the responses that obtained from the stimuli were recorded with Medelec Snergy Amplifier device. The round window ECoG records which were obtained from these 30 patients were compared with the preoperative ABR results. Round window ECoG responses were acquired during operation in 22 patients who had no response to ABR preoperatively. Two patients responded to both preoperative ABR and perioperative round window ECoG. And 7 patients had no preoperative ABR and Discussion:perioperative round window ECoG responses. Our outcome data shows that round window ECoG is more sensitive than ABR while deciding for cochlear implantation. The results are statistically significant. -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid.