The Active Cochlea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiological Mechanisms and Functional Hearing Consequences of Auditory Neuropathy
doi:10.1093/brain/awv270 BRAIN 2015: 138; 3141–3158 | 3141 REVIEW ARTICLE Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy Gary Rance1 and Arnold Starr2 The effects of inner ear abnormality on audibility have been explored since the early 20th century when sound detection measures were first used to define and quantify ‘hearing loss’. The development in the 1970s of objective measures of cochlear hair cell Downloaded from function (cochlear microphonics, otoacoustic emissions, summating potentials) and auditory nerve/brainstem activity (auditory brainstem responses) have made it possible to distinguish both synaptic and auditory nerve disorders from sensory receptor loss. This distinction is critically important when considering aetiology and management. In this review we address the clinical and pathophysiological features of auditory neuropathy that distinguish site(s) of dysfunction. We describe the diagnostic criteria for: (i) presynaptic disorders affecting inner hair cells and ribbon synapses; (ii) postsynaptic disorders affecting unmyelinated auditory http://brain.oxfordjournals.org/ nerve dendrites; (iii) postsynaptic disorders affecting auditory ganglion cells and their myelinated axons and dendrites; and (iv) central neural pathway disorders affecting the auditory brainstem. We review data and principles to identify treatment options for affected patients and explore their benefits as a function of site of lesion. 1 Department of Audiology and Speech Pathology, The University of Melbourne, -

Perforated Eardrum
Vinod K. Anand, MD, FACS Nose and Sinus Clinic Perforated Eardrum A perforated eardrum is a hole or rupture m the eardrum, a thin membrane which separated the ear canal and the middle ear. The medical term for eardrum is tympanic membrane. The middle ear is connected to the nose by the eustachian tube. A perforated eardrum is often accompanied by decreased hearing and occasional discharge. Paih is usually not persistent. Causes of Eardrum Perforation The causes of perforated eardrum are usually from trauma or infection. A perforated eardrum can occur: if the ear is struck squarely with an open hand with a skull fracture after a sudden explosion if an object (such as a bobby pin, Q-tip, or stick) is pushed too far into the ear canal. as a result of hot slag (from welding) or acid entering the ear canal Middle ear infections may cause pain, hearing loss and spontaneous rupture (tear) of the eardrum resulting in a perforation. In this circumstance, there may be infected or bloody drainage from the ear. In medical terms, this is called otitis media with perforation. On rare occasions a small hole may remain in the eardrum after a previously placed P.E. tube (pressure equalizing) either falls out or is removed by the physician. Most eardrum perforations heal spontaneously within weeks after rupture, although some may take up to several months. During the healing process the ear must be protected from water and trauma. Those eardrum perforations which do not heal on their own may require surgery. Effects on Hearing from Perforated Eardrum Usually, the larger the perforation, the greater the loss of hearing. -

A Pictorial Review of Round Window Pathologies
REVIEW ARTICLE The Forgotten Second Window: A Pictorial Review of Round Window Pathologies J.C. Benson, F. Diehn, T. Passe, J. Guerin, V.M. Silvera, M.L. Carlson, and J. Lane ABSTRACT SUMMARY: The round window serves to decompress acoustic energy that enters the cochlea via stapes movement against the oval window. Any inward motion of the oval window via stapes vibration leads to outward motion of the round window. Occlusion of the round window is a cause of conductive hearing loss because it increases the resistance to sound energy and con- sequently dampens energy propagation. Because the round window niche is not adequately evaluated by otoscopy and may be incompletely exposed during an operation, otologic surgeons may not always correctly identify associated pathology. Thus, radiol- ogists play an essential role in the identification and classification of diseases affecting the round window. The purpose of this review is to highlight the developmental, acquired, neoplastic, and iatrogenic range of pathologies that can be encountered in round window dysfunction. ABBREVIATIONS: LO 4 labyrinthitis ossificans; RW 4 round window he round window (RW) serves as a boundary between the considerations of the round window that can be encoun- Tbasal turn of the cochlea anteriorly and the round win- tered on imaging are reviewed. dow niche posteriorly.1 It, along with the oval window, is 1 of 2 natural openings between the inner and middle ear. The Physiology and Functionality “ ” round window is often overshadowed by the first window The inner ear “windows” refer to openings in the otic capsule “ ” (the oval window) and pathologic third windows (eg, that connect the fluid in the inner ear to either the middle ear or superior semicircular canal dehiscence). -

The Standing Acoustic Wave Principle Within the Frequency Analysis Of
inee Eng ring al & ic d M e e d Misun, J Biomed Eng Med Devic 2016, 1:3 m i o c i a B l D f o e v DOI: 10.4172/2475-7586.1000116 l i a c n e r s u o Journal of Biomedical Engineering and Medical Devices J ISSN: 2475-7586 Review Article Open Access The Standing Acoustic Wave Principle within the Frequency Analysis of Acoustic Signals in the Cochlea Vojtech Misun* Department of Solid Mechanics, Mechatronics and Biomechanics, Brno University of Technology, Brno, Czech Republic Abstract The organ of hearing is responsible for the correct frequency analysis of auditory perceptions coming from the outer environment. The article deals with the principles of the analysis of auditory perceptions in the cochlea only, i.e., from the overall signal leaving the oval window to its decomposition realized by the basilar membrane. The paper presents two different methods with the function of the cochlea considered as a frequency analyzer of perceived acoustic signals. First, there is an analysis of the principle that cochlear function involves acoustic waves travelling along the basilar membrane; this concept is one that prevails in the contemporary specialist literature. Then, a new principle with the working name “the principle of standing acoustic waves in the common cavity of the scala vestibuli and scala tympani” is presented and defined in depth. According to this principle, individual structural modes of the basilar membrane are excited by continuous standing waves of acoustic pressure in the scale tympani. Keywords: Cochlea function; Acoustic signals; Frequency analysis; The following is a description of the theories in question: Travelling wave principle; Standing wave principle 1. -

The Round Window Region and Contiguous Areas: Endoscopic Anatomy and Surgical Implications
Eur Arch Otorhinolaryngol DOI 10.1007/s00405-014-2923-8 OTOLOGY The round window region and contiguous areas: endoscopic anatomy and surgical implications Daniele Marchioni · Matteo Alicandri-Ciufelli · David D. Pothier · Alessia Rubini · Livio Presutti Received: 16 October 2013 / Accepted: 28 January 2014 © Springer-Verlag Berlin Heidelberg 2014 Abstract The round window region is a critical area of window region. Exact anatomical knowledge of this region the middle ear; the aim of this paper is to describe its anat- can have important advantages during surgery, since some omy from an endoscopic perspective, emphasizing some pathology can invade inside cavities or tunnels otherwise structures, the knowledge of which could have important not seen by instrumentation that produces a straight-line implications during surgery, as well as to evaluate what view (e.g. microscope). involvement cholesteatoma may have with these structures. Retrospective review of video recordings of endoscopic Keywords Retrotympanum · Endoscopic ear surgery · ear surgeries and retrospective database review were con- Cholesteatoma · Middle ear anatomy · Round window ducted in Tertiary university referral center. Videos from endoscopic middle ear procedures carried out between June 2010 and September 2012 and stored in a shared data- Introduction base were reviewed retrospectively. Surgeries in which an endoscopic magnification of the round window region and The surgical management of cholesteatoma is still a contro- the inferior retrotympanum area was performed intraop- versial issue. Endoscopic instrumentation, techniques and eratively were included in the study. Involvement by cho- knowledge have improved considerably over the last few lesteatoma of those regions was also documented based years, and we believe that, in the future, endoscopic surgi- on information obtained from the surgical database. -

Morphological and Functional Changes in a New Animal Model Of
Laboratory Investigation (2013) 93, 1001–1011 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Morphological and functional changes in a new animal model of Me´nie`re’s disease Naoya Egami1, Akinobu Kakigi1, Takashi Sakamoto1, Taizo Takeda2, Masamitsu Hyodo2 and Tatsuya Yamasoba1 The purpose of this study was to clarify the underlying mechanism of vertiginous attacks in Me´nie`re’s disease (MD) while obtaining insight into water homeostasis in the inner ear using a new animal model. We conducted both histopatho- logical and functional assessment of the vestibular system in the guinea-pig. In the first experiment, all animals were maintained 1 or 4 weeks after electrocauterization of the endolymphatic sac of the left ear and were given either saline or desmopressin (vasopressin type 2 receptor agonist). The temporal bones from both ears were harvested and the extent of endolymphatic hydrops was quantitatively assessed. In the second experiment, either 1 or 4 weeks after surgery, animals were assessed for balance disorders and nystagmus after the administration of saline or desmopressin. In the first experiment, the proportion of endolymphatic space in the cochlea and the saccule was significantly greater in ears that survived for 4 weeks after surgery and were given desmopressin compared with other groups. In the second experiment, all animals that underwent surgery and were given desmopressin showed spontaneous nystagmus and balance disorder, whereas all animals that had surgery but without desmopressin administration were asymptomatic. Our animal model induced severe endolymphatic hydrops in the cochlea and the saccule, and showed episodes of balance disorder along with spontaneous nystagmus. -

Eardrum Regeneration: Membrane Repair
OUTLINE Watch an animation at: Infographic: go.nature.com/2smjfq8 Pages S6–S7 EARDRUM REGENERATION: MEMBRANE REPAIR Can tissue engineering provide a cheap and convenient alternative to surgery for eardrum repair? DIANA GRADINARU he eardrum, or tympanic membrane, forms the interface between the outside world and the delicate bony structures Tof the middle ear — the ossicles — that conduct sound vibrations to the inner ear. At just a fraction of a millimetre thick and held under tension, the membrane is perfectly adapted to transmit even the faintest of vibrations. But the qualities that make the eardrum such a good conductor of sound come at a price: fra- gility. Burst eardrums are a major cause of conductive hearing loss — when sounds can’t pass from the outer to the inner ear. Most burst eardrums are caused by infections or trauma. The vast majority heal on their own in about ten days, but for a small proportion of people the perforation fails to heal natu- rally. These chronic ruptures cause conductive hearing loss and group (S. Kanemaru et al. Otol. Neurotol. 32, 1218–1223; 2011). increase the risk of middle ear infections, which can have serious In a commentary in the same journal, Robert Jackler, a head complications. and neck surgeon at Stanford University, California, wrote that, Surgical intervention is the only option for people with ear- should the results be replicated, the procedure represents “poten- drums that won’t heal. Tympanoplasty involves collecting graft tially the greatest advance in otology since the invention of the material from the patient to use as a patch over the perforation. -
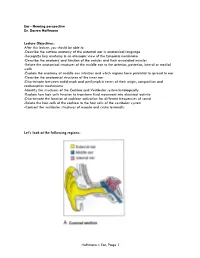
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

CONGENITAL MALFORMATIONS of the INNER EAR Malformaciones Congénitas Del Oído Interno
topic review CONGENITAL MALFORMATIONS OF THE INNER EAR Malformaciones congénitas del oído interno. Revisión de tema Laura Vanessa Ramírez Pedroza1 Hernán Darío Cano Riaño2 Federico Guillermo Lubinus Badillo2 Summary Key words (MeSH) There are a great variety of congenital malformations that can affect the inner ear, Ear with a diversity of physiopathologies, involved altered structures and age of symptom Ear, inner onset. Therefore, it is important to know and identify these alterations opportunely Hearing loss Vestibule, labyrinth to lower the risks of all the complications, being of great importance, among others, Cochlea the alterations in language development and social interactions. Magnetic resonance imaging Resumen Existe una gran variedad de malformaciones congénitas que pueden afectar al Palabras clave (DeCS) oído interno, con distintas fisiopatologías, diferentes estructuras alteradas y edad Oído de aparición de los síntomas. Por lo anterior, es necesario conocer e identificar Oído interno dichas alteraciones, con el fin de actuar oportunamente y reducir el riesgo de las Pérdida auditiva Vestíbulo del laberinto complicaciones, entre otras —de gran importancia— las alteraciones en el área del Cóclea lenguaje y en el ámbito social. Imagen por resonancia magnética 1. Epidemiology • Hyperbilirubinemia Ear malformations occur in 1 in 10,000 or 20,000 • Respiratory distress from meconium aspiration cases (1). One in every 1,000 children has some degree • Craniofacial alterations (3) of sensorineural hearing impairment, with an average • Mechanical ventilation for more than five days age at diagnosis of 4.9 years. The prevalence of hearing • TORCH Syndrome (4) impairment in newborns with risk factors has been determined to be 9.52% (2). -

Measuring Cochlear Duct Length – a Historical Analysis of Methods and Results Robert W
Koch et al. Journal of Otolaryngology - Head and Neck Surgery (2017) 46:19 DOI 10.1186/s40463-017-0194-2 REVIEW Open Access Measuring Cochlear Duct Length – a historical analysis of methods and results Robert W. Koch1*, Hanif M. Ladak1,2,3,4†, Mai Elfarnawany2† and Sumit K. Agrawal1,2,4,5† Abstract Background: Cochlear Duct Length (CDL) has been an important measure for the development and advancement of cochlear implants. Emerging literature has shown CDL can be used in preoperative settings to select the proper sized electrode and develop customized frequency maps. In order to improve post-operative outcomes, and develop new electrode technologies, methods of measuring CDL must be validated to allow usage in the clinic. Purpose: The purpose of this review is to assess the various techniques used to calculate CDL and provide the reader with enough information to make an informed decision on how to conduct future studies measuring the CDL. Results: The methods to measure CDL, the modality used to capture images, and the location of the measurement have all changed as technology evolved. With recent popularity and advancement in computed tomography (CT) imaging in place of histologic sections, measurements of CDL have been focused at the lateral wall (LW) instead of the organ of Corti (OC), due to the inability of CT to view intracochlear structures. After analyzing results from methods such as directly measuring CDL from histology, indirectly reconstructing the shape of the cochlea, and determining CDL based on spiral coefficients, it was determined the three dimensional (3D) reconstruction method is the most reliable method to measure CDL. -

Special Ear Problem (Worksheet)
Special Ear Problem (Worksheet) anvil hammer oval window Extenal auditory canal Cochlea Ear drum stirrip organ of corti Round window Basilar membrane A) Label on the drawing above: 1. External auditory canal 2. ear drum (tympanic membrane) 3. hammer (maleus) 4. anvil (incus) 5. stirrup (stapes) 6. cochlea 7. oval window 8. round window 9. basilar membrane 10. organ of Corti B) In one or two sentences, what is the function of the outer ear The outer ear collects sound and guides it to the eardrum. C) The external auditory canal functions like a 2.5 cm closed pipe. What are the first two resonances of this pipe and how do these explain features of Figure 12-7 on page 354 of your book (Giancoli)? The first two resonances are about 3500 Hz --- the frequency where your ear is most sensitive --- and 10,5000 --- which corresponds to kinks in the curves of Fig. 12-7. D)What is the boundary between the outer ear and the middle ear? The Eardrum E) In one or two sentences, what is the function of the middle ear? The middle ear takes the pressure wave coming from the (large area) eardrum and “amplifies” the pressure by about 40 to generate a wave in the fluid of the cochlea through the (small area) oval window. F) What is the boundary between the middle ear and the inner ear? The oval window. G) In one or two sentences, what is the function of the inner ear? The inner ear generates electrical signals from the (liquid) pressure waves generated at the oval window.