37 Histopathology of Irritant Contact Dermatitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Pathomorpholog.Pdf
Ukrаiniаn Medicаl Stomаtologicаl Аcаdemy THE DEPАRTАMENT OF PАTHOLOGICАL АNАTOMY WITH SECTIONSL COURSE MАNUАL for the foreign students GENERАL PАTHOMORPHOLOGY Poltаvа-2020 УДК:616-091(075.8) ББК:52.5я73 COMPILERS: PROFESSOR I. STАRCHENKO ASSOCIATIVE PROFESSOR O. PRYLUTSKYI АSSISTАNT A. ZADVORNOVA ASSISTANT D. NIKOLENKO Рекомендовано Вченою радою Української медичної стоматологічної академії як навчальний посібник для іноземних студентів – здобувачів вищої освіти ступеня магістра, які навчаються за спеціальністю 221 «Стоматологія» у закладах вищої освіти МОЗ України (протокол №8 від 11.03.2020р) Reviewers Romanuk A. - MD, Professor, Head of the Department of Pathological Anatomy, Sumy State University. Sitnikova V. - MD, Professor of Department of Normal and Pathological Clinical Anatomy Odessa National Medical University. Yeroshenko G. - MD, Professor, Department of Histology, Cytology and Embryology Ukrainian Medical Dental Academy. A teaching manual in English, developed at the Department of Pathological Anatomy with a section course UMSA by Professor Starchenko II, Associative Professor Prylutsky OK, Assistant Zadvornova AP, Assistant Nikolenko DE. The manual presents the content and basic questions of the topic, practical skills in sufficient volume for each class to be mastered by students, algorithms for describing macro- and micropreparations, situational tasks. The formulation of tests, their number and variable level of difficulty, sufficient volume for each topic allows to recommend them as preparation for students to take the licensed integrated exam "STEP-1". 2 Contents p. 1 Introduction to pathomorphology. Subject matter and tasks of 5 pathomorphology. Main stages of development of pathomorphology. Methods of pathanatomical diagnostics. Methods of pathomorphological research. 2 Morphological changes of cells as response to stressor and toxic damage 8 (parenchimatouse / intracellular dystrophies). -

Paraneoplastic Syndrome Presenting As Giant Porokeratosis in a Patient with Nasopharyngeal Cancer
Paraneoplastic Syndrome Presenting As Giant Porokeratosis in A Patient with Nasopharyngeal Cancer Fitri Azizah, Sonia Hanifati, Sri Adi Sularsito, Lili Legiawati, Shannaz Nadia Yusharyahya, Rahadi Rihatmadja Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo National General Hospital Keywords: porokeratosis, giant porokeratosis, paraneoplastic syndrome, nasopharyngeal Abstract: Giant porokeratosis is a rare condition in which the hyperkeratotic plaques of porokeratosis reach up to 20 cm in diameter. Porokeratosis is characterized clinically by hyperkeratotic papules or plaques with a thread-like elevated border. Although rare, porokeratosis has been reported in conjunction with malignancies suggesting a paraneoplastic nature. Associated malignancies reported were hematopoietic, hepatocellular, and cholangiocarcinoma. We report a case of giant porokeratosis in a patient with nasopharyngeal cancer responding to removal of the primary cancer by chemoradiotherapy. 1 INTRODUCTION regress completely after the treatment of malignancy, suggestive of paraneoplastic syndrome. Porokeratosis is a chronic progressive disorder of keratinization, characterized by hyperkeratotic papules or plaques surrounded by a thread-like 2 CASE elevated border corresponds to a typical histologic hallmark, the cornoid lamella . O regan, 2012) There Mr. SS, 68-year-old, was referred for evaluation of are at least six clinical variants of porokeratosis pruritic, slightly erythematous plaques with raised, recognized with known genetic disorder.1 Some hyperpigmented border of one and a half year clinical variant of porokeratosis has been reported in duration on the extensor surface of both legs. The the setting of immunosuppressive conditions, organ lesions shown minimal response to potent topical transplantation, use of systemic corticosteroids, and corticosteroids and phototherapy given during the infections, suggesting that impaired immunity may last 8 months in another hospital. -

Features of Reactive White Lesions of the Oral Mucosa
Head and Neck Pathology (2019) 13:16–24 https://doi.org/10.1007/s12105-018-0986-3 SPECIAL ISSUE: COLORS AND TEXTURES, A REVIEW OF ORAL MUCOSAL ENTITIES Frictional Keratosis, Contact Keratosis and Smokeless Tobacco Keratosis: Features of Reactive White Lesions of the Oral Mucosa Susan Müller1 Received: 21 September 2018 / Accepted: 2 November 2018 / Published online: 22 January 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract White lesions of the oral cavity are quite common and can have a variety of etiologies, both benign and malignant. Although the vast majority of publications focus on leukoplakia and other potentially malignant lesions, most oral lesions that appear white are benign. This review will focus exclusively on reactive white oral lesions. Included in the discussion are frictional keratoses, irritant contact stomatitis, and smokeless tobacco keratoses. Leukoedema and hereditary genodermatoses that may enter in the clinical differential diagnoses of frictional keratoses including white sponge nevus and hereditary benign intraepithelial dyskeratosis will be reviewed. Many products can result in contact stomatitis. Dentrifice-related stomatitis, contact reactions to amalgam and cinnamon can cause keratotic lesions. Each of these lesions have microscopic findings that can assist in patient management. Keywords Leukoplakia · Frictional keratosis · Smokeless tobacco keratosis · Stomatitis · Leukoedema · Cinnamon Introduction white lesions including infective and non-infective causes will be discussed -

Review an Overview of the Pale and Clear Cells of the Nipple Epidermis
Histol Histopathol (2009) 24: 367-376 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology Review An overview of the pale and clear cells of the nipple epidermis M.F. Garijo, D. Val and J.F. Val-Bernal Department of Anatomical Pathology, Marqués de Valdecilla University Hospital, Medical Faculty, University of Cantabria, Santander, Spain Summary. The stratified squamous epithelium of the exterior. In the non-lactating breast these duct openings nipple-areola complex may contain pale or clear cells are usually filled with plugs of keratin. The nipple and including: Paget’s disease cells (PDCs), Toker cells areola are covered by keratinizing stratified squamous (TCs), and so-called clear cells (CCs). Paget’s disease is epithelium similar to that seen in the epidermis an uncommon presentation of breast carcinoma. PDCs elsewhere in the body. The collecting ducts show a are large, atypical, have abundant, pale-staining double epithelial and myoepithelial lining. The cytoplasm that may contain mucin secretion vacuoles epithelium is columnar and the myoepithelial cells lie and bulky heterochromatic nuclei. They are commonly between the epithelial layer and the basal lamina. A concentrated along the basal layer and stain for EMA, cross section of the major ducts shows an irregular, CAM5.2, cytokeratin 7, and HER2/neu oncoprotein. TCs pleated or serrated outline and an investment with are bland cells with roundish and scant chromatin nuclei. muscular tissue. The areola dermis contains numerous They are found incidentally and are reactive for EMA, sebaceous glands. Some of them open directly onto the CAM5.2, and cytokeratin 7, but show negativity for surface, whereas others drain into a collecting duct or HER2/neu oncoprotein. -

An Update on the Biology and Management of Dyskeratosis Congenita and Related Telomere Biology Disorders
Expert Review of Hematology ISSN: 1747-4086 (Print) 1747-4094 (Online) Journal homepage: https://www.tandfonline.com/loi/ierr20 An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch & Sharon A. Savage To cite this article: Marena R. Niewisch & Sharon A. Savage (2019) An update on the biology and management of dyskeratosis congenita and related telomere biology disorders, Expert Review of Hematology, 12:12, 1037-1052, DOI: 10.1080/17474086.2019.1662720 To link to this article: https://doi.org/10.1080/17474086.2019.1662720 Accepted author version posted online: 03 Sep 2019. Published online: 10 Sep 2019. Submit your article to this journal Article views: 146 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ierr20 EXPERT REVIEW OF HEMATOLOGY 2019, VOL. 12, NO. 12, 1037–1052 https://doi.org/10.1080/17474086.2019.1662720 REVIEW An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch and Sharon A. Savage Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA ABSTRACT ARTICLE HISTORY Introduction: Telomere biology disorders (TBDs) encompass a group of illnesses caused by germline Received 14 June 2019 mutations in genes regulating telomere maintenance, resulting in very short telomeres. Possible TBD Accepted 29 August 2019 manifestations range from complex multisystem disorders with onset in childhood such as dyskeratosis KEYWORDS congenita (DC), Hoyeraal-Hreidarsson syndrome, Revesz syndrome and Coats plus to adults presenting Telomere; dyskeratosis with one or two DC-related features. -

Wednesday Slide Conference 2008-2009
PROCEEDINGS DEPARTMENT OF VETERINARY PATHOLOGY WEDNESDAY SLIDE CONFERENCE 2008-2009 ARMED FORCES INSTITUTE OF PATHOLOGY WASHINGTON, D.C. 20306-6000 2009 ML2009 Armed Forces Institute of Pathology Department of Veterinary Pathology WEDNESDAY SLIDE CONFERENCE 2008-2009 100 Cases 100 Histopathology Slides 249 Images PROCEEDINGS PREPARED BY: Todd Bell, DVM Chief Editor: Todd O. Johnson, DVM, Diplomate ACVP Copy Editor: Sean Hahn Layout and Copy Editor: Fran Card WSC Online Management and Design Scott Shaffer ARMED FORCES INSTITUTE OF PATHOLOGY Washington, D.C. 20306-6000 2009 ML2009 i PREFACE The Armed Forces Institute of Pathology, Department of Veterinary Pathology has conducted a weekly slide conference during the resident training year since 12 November 1953. This ever- changing educational endeavor has evolved into the annual Wednesday Slide Conference program in which cases are presented on 25 Wednesdays throughout the academic year and distributed to 135 contributing military and civilian institutions from around the world. Many of these institutions provide structured veterinary pathology resident training programs. During the course of the training year, histopathology slides, digital images, and histories from selected cases are distributed to the participating institutions and to the Department of Veterinary Pathology at the AFIP. Following the conferences, the case diagnoses, comments, and reference listings are posted online to all participants. This study set has been assembled in an effort to make Wednesday Slide Conference materials available to a wider circle of interested pathologists and scientists, and to further the education of veterinary pathologists and residents-in-training. The number of histopathology slides that can be reproduced from smaller lesions requires us to limit the number of participating institutions. -

Inflammatory Skin Disease Every Pathologist Should Know
Inflammatory skin disease every pathologist should know Steven D. Billings Cleveland Clinic [email protected] General Concepts • Pattern recognition – Epidermal predominant vs. dermal predominant • Epidermal changes trump dermal changes – Distribution of the inflammatory infiltrate • Superficial vs. superficial and deep • Location: perivascular, interstitial, nodular – Nature of inflammatory infiltrate • Mononuclear (lymphocytes and histiocytes) • Mixed (mononuclear and granulocytes) • Granulocytic • Correlation with clinical presentation • Never diagnose “chronic nonspecific dermatitis” Principle Patterns: Epidermal Changes Predominant • Spongiotic pattern • Psoriasiform pattern – Spongiotic and psoriasiform often co-exist • Interface pattern – Basal vacuolization • Perivascular infiltrate or • Lichenoid infiltrate Principle Patterns: Dermal Changes Predominant • Superficial perivascular • Superficial and deep perivascular • Interstitial pattern – Palisading granulomatous – Nodular and diffuse • Sclerosing pattern • Panniculitis • Bullous disease • Miscellaneous Spongiotic Dermatitis • Three phases – Acute – Subacute – Chronic • Different but overlapping histologic features Spongiotic Dermatitis • Acute spongiotic dermatitis – Normal “basket-weave” stratum corneum – Pale keratinocytes – Spongiosis – Spongiotic vesicles (variable) – Papillary dermal edema – Variable superficial perivascular infiltrate of lymphocytes often with some eosinophils – Rarely biopsied in acute phase Spongiotic Dermatitis • Subacute spongiotic dermatitis – Parakeratosis -
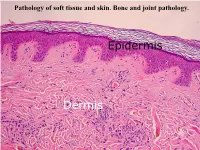
Pathology of Soft Tissue and Skin. Bone and Joint Pathology. Pathology of Soft Tissue and Skin
Pathology of soft tissue and skin. Bone and joint pathology. Pathology of soft tissue and skin. Bone and joint pathology. I.Microspecimens: № 188. Capillary hemangioma. (H.E. stain). Indications: 1. Epidermis. 2. Dermis 3. Spindle cells arranged compactly with spaces containing blood. 4. Reduced connective stroma. In the microspecimen is presented a well-defined subepidermal tumoral node, consisting of proliferating capillary blood vessels, poor loose stroma; epidermis with normal histological structure. Hemangioma is a benign tumor of vascular origin, histological variants are capillary, venous and cavernous hemangioma. It is located mainly in the skin, the mucosa of the gastrointestinal tract, the liver. Capillary hemangioma is the most common benign tumor in children and has a disembryoplazic character, being interpreted as a hamartoma - a tumor from the embryonic tissues. Macroscopically it has the appearance of a red-purple node or plaque. Cutaneous hemangiomas can be complicated by exulceration, bleeding, the association of secondary infection. 3 2 4 1 № 188. Capillary hemangioma. (H.E. stain). № 43. Fibrosarcoma. (H.E. stain). Indications: 1.Epidermis. 2.Dermis. 3.Atypical tumor cells (fibroblast-like). 4.Bundles of collagen fibers. In the skin, under the epidermis there is a rich cellular tumoral node, consisting of predominantly spindle-shaped cells, of the fibroblasts type, arranged in bundles, which intersect in different directions, the tumor has no precise limits, many mitoses, giant cells, foci of necrosis, hemorrhage, stroma is poor. Fibrosarcoma is a malignant tumor, which derives from fibroblasts, may have different degrees of differentiation. It is found in adults between the ages of 40 and 70, located more frequently in the deep tissues of the hip, knee, in the retroperitoneal area. -

Incidental Focal Acantholytic Dyskeratosis in the Setting of Rosacea
Letter to the Editor http://dx.doi.org/10.5021/ad.2013.25.4.518 Incidental Focal Acantholytic Dyskeratosis in the Setting of Rosacea Sang-Yeon Park, Hae Jin Lee, Jae Yong Shin, Sung Ku Ahn Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea Dear Editor: direct immunofluorescence (Fig. 2). In the serial sections, Focal acantholytic dyskeratosis (FAD) was first described we observe the same findings. Differential diagnosis for by Ackerman1 in 1972 as a distinct histopathological pat- the possibility of polymorphous light eruption, systemic tern associated with various cutaneous conditions, and lupus erythematosus, contact dermatitis, and dermatitis with classic histopathological findings including supra- artefacta should be considered. But given these clinical basal clefting, hyperkeratosis and parakeratosis, and the and histopathological features, a diagnosis of rosacea with presence of acantholytic and dyskeratotic cells at the FAD was reached. The patient was then admitted to epidermis. While FAD can be observed in many various hospital for treatment with doxycycline 100 mg and cutaneous lesions including benign and/or malignant antihistamines. After one week, the lesions had remarka- epithelial lesions, fibrohistiocytic lesions, inflammatory bly improved. The patient was then discharged, and lesions, melanocytic and/or follicular lesions2-4. These continued on the same therapeutic regimen for an histopathological findings may also extend into the additional month, bythe time all lesions were nearly surrounding tissues, which often appear to be clinically resolved. normal. A 42-year-old woman was presented to our To date, the etiology of FAD has been attributed to department with multiple erythematous pruritic papules numerous sources including hormones, viral infection, and tiny vesicles on her face. -

Tern, It Was Felt of Use to Record the Experience in This Matter
PERSISTENT "INSECT BITES" (DERMAL EOSINOPHILIC GRANU- LOMAS) SIMULATING LYMPHOBLASTOMAS, HISTIOCYTOSES, AND SQUAMOUS CELL CARCINOMAS * ARrHUR C. ALLEN, MD. t (From the Army instute of Pathology, Washingtox 25, D.C.) In 1942, opportunity was afforded at the Army Institute of Pathol- ogy to review the histologic slides of a lesion said to have been pro- duced by a tick bite. The microscopic sections seemed at the time indistinguishable from mycosis fungoides or Hodgkin's disee, espe- cialy in view of the presence of multiple lesions in the patient. How- ever, following the study of the cutaneous reactions to arthropods (ticks, mosquitoes, and chiggers), it was quickly appreciated that not only were these diagnoses of neoplasia wrong but that the misinterpretation of these reactions was a common and serious error.' The errors in- volved the misconstruction not only of the dermal reaction but also of the epidermal changes. The latter response was confused with squa- mous cell carcinoma; the dermal infitrate was mistaken for mycosis fungoides, Hodgkin's disease, lymphosacoma, giant follicular lympho- blastoma, and Spiegler-Fendt sarcoid. Undoubtedly the principal rea- son for the failure to attnbute these reactions properly to bites of arachnida and insects was referable to the general impression, despite dear-cut cinical histories, that such reactions last only for days, whereas, in truth, they may persist for as long as 2 years. More re- cently, the problem has been further complicated by introduction into the literature of a lesion called "eosinophilic granuloma of skin," an entity of questionable nosologic justification.' Therefore, because of the major importance of establishing a definitive diagnosis and because of the interest in the pathogenesis of a much mimicked histologic pat- tern, it was felt of use to record the experience in this matter. -

ECZEMA) Shankar Pratap K
Research Article International Ayurvedi c Medical Journal ISSN:2320 5091 A HISTOPATHOLOGICAL STUDY ON LEECH APPLICATION IN THE MANAGEMENT OF VICARCIKA (ECZEMA) Shankar Pratap K. M.1 Rao Dattatreya 2 Sai Prasad 3 1Dept. of Shalya Tantra , Santhigiri Ayurvedic College , Palakkad, Kerala, India 2Dept. of Shalya Tantra, S.V. Ayurvedic College, Tirupati , Andhra Pradesh , India 3Dept. of Pathology, S. V. Medical College, Tirupati, Andhra Pradesh, India ABSTRACT To assess the efficacy of Leech application in the management of Vicarcik a (Eczema) with Histopathological study, the present study with 10 patients having the classical symptoms of Vicarcika, were randomly selected as per the inclusion and exclusion criteria from O.P.D. & I.P.D. sections of Shalya department, S.V. Ayurvedic Hospital, Tirupati. Minimum 4 sittings of Leech application was carried out with seven days interval. Total duration of treatment was 6 weeks. Biopsy samples were collected from the lesion site before and after treatment. Histopathological examination was done by the pathologist. In eczema (dermatitis) the leech application therapy gives excellent response by reducing the inflammatory component, hyperkeratosis, spongiosis, irregular acanthosis and by evoking a granulation tissue response in the dermis and in most of the cases with complete recovery from the lesion. Most of the cases in the study were chronic dermatitis and sebhoric keratosis, almost all local/focal pigmented lesion is totally relieved by leech therapy especially in cases of sebhoric keratos is. In the present study it was found that, leech application evokes significant changes at histological level specifically in reduction of inflammatory component, hyperkeratosis, spongiosis and irregular acanthosis. -

Diffuse Drug-Induced Dermatitis Following Sclerotherapy for Telangiectasias Libby MW, Caitlin WH, Deniz OA and Heller JA*
Case Report iMedPub Journals Journal of Vascular and Endovascular Surgery 2016 http://www.imedpub.com/ Vol.1 No.3:17 DOI: 10.21767/2573-4482.100017 Diffuse Drug-Induced Dermatitis following Sclerotherapy for Telangiectasias Libby MW, Caitlin WH, Deniz OA and Heller JA* Department of Surgery, Johns Hopkins Vein Center, Johns Hopkins Medical Centers, Baltimore, USA *Corresponding author: Jennifer A Heller, 10755 Falls Road, Pavilion 1 Suite 360, Lutherville, MD 21903, Tel: 4105500415; Email: [email protected] Received date: June 14, 2016; Accepted date: July 26, 2016; Published date: August 02, 2016 Copyright: © 2016 Libby MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sclerotherapy is an effective treatment modality for the management of lower extremity telangiectasias. Although localized dermatologic reactions are known complications of this procedure, systemic reactions are rare. Here, we present a case of a diffuse drug eruption following sclerotherapy for the treatment of bilateral lower extremity telangiectasias. Salient clinical and physical exam findings are described. Management strategies for the treatment of drug eruptions are outlined. This is the first case report that we know of describing a diffuse drug eruption associated with sclerotherapy. It is important to recognize the possibility of diffuse drug eruptions secondary to sclerotherapy treatment so that expectant management may be initiated expeditiously in affected patients. Figure 1: Drug eruption following sclerotherapy. The patient was referred for evaluation by a dermatologist, Case Report who initially diagnosed diffuse dermatitis based on clinical A 64-year-old woman with hypertension and bilateral lower exam.