Boards' Fodder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D
Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D. Director of Ethnic Skin Care Voluntary Assistant Professor Miller/University of Miami School of Medicine Department of Dermatology and Cutaneous Surgery What Determines Skin Color? What Determines Skin Color? No significant difference in the number of melanocytes between the races 2000 epidermal melanocytes/mm2 on head and forearm 1000 epidermal melanocytes/mm2 on the rest of the body differences present at birth Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff,K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192-220, New York, NY: McGraw Hill Melanosomes in Black and White Skin Black White Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Nature1969;222:1081-1082 Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192- 220, New York, NY: McGraw Hill Role of Melanin-Advantages Melanin absorbs and scatters energy from UV and visible light to protect epidermal cells from UV damage Disadvantages Inflammation or injury to the skin is almost immediately accompanied by alteration in pigmentation Hyperpigmentation Hypopigmentation Dyschromias Post-Inflammatory hyperpigmentation Acne Melasma Lichen Planus Pigmentosus Progressive Macular Hypomelanosis -

Paraneoplastic Syndrome Presenting As Giant Porokeratosis in a Patient with Nasopharyngeal Cancer
Paraneoplastic Syndrome Presenting As Giant Porokeratosis in A Patient with Nasopharyngeal Cancer Fitri Azizah, Sonia Hanifati, Sri Adi Sularsito, Lili Legiawati, Shannaz Nadia Yusharyahya, Rahadi Rihatmadja Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo National General Hospital Keywords: porokeratosis, giant porokeratosis, paraneoplastic syndrome, nasopharyngeal Abstract: Giant porokeratosis is a rare condition in which the hyperkeratotic plaques of porokeratosis reach up to 20 cm in diameter. Porokeratosis is characterized clinically by hyperkeratotic papules or plaques with a thread-like elevated border. Although rare, porokeratosis has been reported in conjunction with malignancies suggesting a paraneoplastic nature. Associated malignancies reported were hematopoietic, hepatocellular, and cholangiocarcinoma. We report a case of giant porokeratosis in a patient with nasopharyngeal cancer responding to removal of the primary cancer by chemoradiotherapy. 1 INTRODUCTION regress completely after the treatment of malignancy, suggestive of paraneoplastic syndrome. Porokeratosis is a chronic progressive disorder of keratinization, characterized by hyperkeratotic papules or plaques surrounded by a thread-like 2 CASE elevated border corresponds to a typical histologic hallmark, the cornoid lamella . O regan, 2012) There Mr. SS, 68-year-old, was referred for evaluation of are at least six clinical variants of porokeratosis pruritic, slightly erythematous plaques with raised, recognized with known genetic disorder.1 Some hyperpigmented border of one and a half year clinical variant of porokeratosis has been reported in duration on the extensor surface of both legs. The the setting of immunosuppressive conditions, organ lesions shown minimal response to potent topical transplantation, use of systemic corticosteroids, and corticosteroids and phototherapy given during the infections, suggesting that impaired immunity may last 8 months in another hospital. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
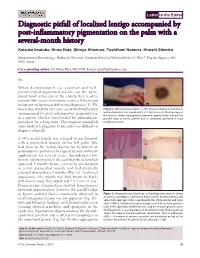
Diagnostic Pitfall of Localized Lentigo Accompanied by Post-Inflammatory
Letter to the Editor Diagnostic pitfall of localized lentigo accompanied by post-inflammatory pigmentation on the palm with a several-month history Keisuke Imafuku, Hiroo Hata, Shinya Kitamura, Toshifumi Nomura, Hiroshi Shimizu Department of Dermatology, Hokkaido University Graduate School of MedicineNorth 15, West 7, Kita-ku, Sapporo 060- 8638, Japan Corresponding author: Dr. Hiroo Hata, MD, PhD, E-mail: [email protected] Sir, When dermatologists see acquired and well- circumscribed pigmented macules on the palm, dorsal hand or forearm of the elderly, they tend to consider blue nevus, hematoma, nevus cell nevus and malignant melanoma as differential diagnoses [1]. We a b herein described the very rare case of localized lentigo Figure 1: Clinical manifestations. a. The macule is brown to dark black, accompanied by post-inflammatory pigmentation well-demarcated, flat, round and 3 x 4 mm in size. b. Dermoscopy of the macule shows homogenous brownish pigmentation without the in a patient who has been treated for palmoplanter parallel ridge or furrow pattern that is commonly observed in acral pustulosis for a long time. This eruption mimicked lentiginous lesion some kinds of lentiginous lesion and it was difficult to diagnose clinically. A 59-year-old female was referred to our hospital with a pigmented macule on her left palm. She had been to the family doctor for treatment of palmoplanter pustulosis by topical steroid ointment application for several years. According to the a history-taking interview, she said that the lesion had b appeared 5 months before referral to our hospital as a tiny pigmented macule and had gradually enlarged during those 5 months (Fig. -

Features of Reactive White Lesions of the Oral Mucosa
Head and Neck Pathology (2019) 13:16–24 https://doi.org/10.1007/s12105-018-0986-3 SPECIAL ISSUE: COLORS AND TEXTURES, A REVIEW OF ORAL MUCOSAL ENTITIES Frictional Keratosis, Contact Keratosis and Smokeless Tobacco Keratosis: Features of Reactive White Lesions of the Oral Mucosa Susan Müller1 Received: 21 September 2018 / Accepted: 2 November 2018 / Published online: 22 January 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract White lesions of the oral cavity are quite common and can have a variety of etiologies, both benign and malignant. Although the vast majority of publications focus on leukoplakia and other potentially malignant lesions, most oral lesions that appear white are benign. This review will focus exclusively on reactive white oral lesions. Included in the discussion are frictional keratoses, irritant contact stomatitis, and smokeless tobacco keratoses. Leukoedema and hereditary genodermatoses that may enter in the clinical differential diagnoses of frictional keratoses including white sponge nevus and hereditary benign intraepithelial dyskeratosis will be reviewed. Many products can result in contact stomatitis. Dentrifice-related stomatitis, contact reactions to amalgam and cinnamon can cause keratotic lesions. Each of these lesions have microscopic findings that can assist in patient management. Keywords Leukoplakia · Frictional keratosis · Smokeless tobacco keratosis · Stomatitis · Leukoedema · Cinnamon Introduction white lesions including infective and non-infective causes will be discussed -

Iris Mammillations: Significance and Associations
IRIS MAMMILLATIONS: SIGNIFICANCE AND ASSOCIATIONS 2 l NICOLA K. RAGGEL2, 1. ACHESON and A. LINN MURPHREE Los Angeles and London SUMMARY mammiform (nipple- or teat-like) protuberances. Iris mammillations are rarely described, distinctive Iris mammillations are an occasional finding with few previous reports. They are most commonly villiform protuberances that can cover the iris. In the l--6 majority of reported cases they are unilateral and found in association with melanosis oculi, with or sporadic, and are seen in association with oculodermal without periocular skin involvement in a naevus of melanosis. In past literature and current clinical Ota. They are thus often less precisely referred to as practice they are frequently confused with tbe iris iris melanosis, a term which should best be reserved nodules seen in neurofibromatosis type 1. Their clinical for increased pigmentation of the iris, irrespective of significance is not established, although it has been the presence of iris elevations overlying the pigmen 7 suggested that iris mammillations may be an external ted areas. This is supported by the rare descriptions sign of ocular hypertension or intraocular malignancy. of iris elevations in the absence of any increased iris We report a series of 9 patients between the ages of 3 pigmentation?,8 and 28 years with iris mammillations. The mammilla Iris mammillations are usually unilateral, often tions appear as regularly spaced, deep brown, smooth, presenting as heterochromia iridis. Occasional bilat 7 conical elevations on the iris, of uniform height or eral cases have been described. ,8 Iris mammillations increasing in height as the pupil margin is approached. -

Unexplained White Spots on Your Skin Could Be Caused by Aging
Unexplained White Spots on Your Skin Could Be Caused by Aging By GERRIE SUMMERS. June 12, 2019 Many of us take great pains to make sure our skin is clear and pristine. (At Byrdie HQ, we've got the robust skincare regimens to prove it.) So, anytime something out of the ordinary pops up that we don't automatically know how to deal with, things can reach panic mode pretty quickly. For example, age spots. And we're not just talking about dark sunspots; we've got a pretty good handle on those. We're talking little white spots, which can look a bit like confetti or white freckles. "It is a little- known fact that sun damage causes not only brown spots, but also white spots," says Dr. Anna Guanche, board-certified dermatologist and celebrity beauty expert at Bella Skin Institute. "I liken them to 'gray hairs' in the skin." More than likely, these are a result of a harmless condition called Idiopathic Guttate Hypomelanosis (IGH). "IGH is a skin condition characterized by multiple round [or] oval white spots that are usually flat. It is very commonly found on the arms and legs of patients over 50," says Dr. Marla Diakow of Schweiger Dermatology Group in Garden City, NY. "[It's] a completely benign entity, but often one of cosmetic concern to patients." These white spots occur due to localized loss of pigmentation of the skin. Read on to get the full scoop on this condition and how it can be treated. How to Treat IGH If you begin to notice unexplained white spots, you should visit your dermatologist ASAP to rule out other conditions that have similar characteristics. -

Hutchinson's Melanotic Freckle
Hutchiso’s elaotic freckle Also known as Lentigo maligna What is lentigo maligna? Lentigo maligna is an early form of melanoma. In lentigo maligna the cancer cells are confined to the upper layer of the skin (epidermis). When the cancer cells spread deeper into the skin (to dermis) it is called lentigo maligna melanoma. Lentigo maligna occurs most commonly in sun damaged areas such as the face and neck in fair skinned people over the age of 60. The lesion grows slowly in size over a number of years. Melanoma is a potentially lethal disease and lentigo maligna should be diagnosed and excised as soon as possible. What causes lentigo maligna? The cause of lentigo maligna is sun exposure or solarium use. Factors that predispose a person to developing lentigo maligna or associated condition include: . chronic sun damaged/solar-induced skin damage . fair skin complexion . male gender . a personal history of non melanoma skin cancer and precancerous lesions . older individuals (those between 60 to 80 years are most commonly affected) What does lentigo maligna look like? Lentigo maligna commonly looks like a freckle, age spot, sun spot or brown patch that slowly changes shape and grows in size. The spot may be large in size, irregularly shaped with a smooth surface, and of multiple shades of brown and sometimes other colours. Thickening of part of the lesion, increasing number of colours, ulceration or bleeding can be markers that the lesion is changing into a lentigo maligna melanoma. How is lentigo maligna diagnosed? Lentigo maligna is diagnosed clinically by a dermatologist, sometimes with the help of a dermatoscope (a tool used to magnify and look closely at skin moles). -

Pigmented Contact Dermatitis and Chemical Depigmentation
18_319_334* 05.11.2005 10:30 Uhr Seite 319 Chapter 18 Pigmented Contact Dermatitis 18 and Chemical Depigmentation Hideo Nakayama Contents ca, often occurs without showing any positive mani- 18.1 Hyperpigmentation Associated festations of dermatitis such as marked erythema, with Contact Dermatitis . 319 vesiculation, swelling, papules, rough skin or scaling. 18.1.1 Classification . 319 Therefore, patients may complain only of a pigmen- 18.1.2 Pigmented Contact Dermatitis . 320 tary disorder, even though the disease is entirely the 18.1.2.1 History and Causative Agents . 320 result of allergic contact dermatitis. Hyperpigmenta- 18.1.2.2 Differential Diagnosis . 323 tion caused by incontinentia pigmenti histologica 18.1.2.3 Prevention and Treatment . 323 has often been called a lichenoid reaction, since the 18.1.3 Pigmented Cosmetic Dermatitis . 324 presence of basal liquefaction degeneration, the ac- 18.1.3.1 Signs . 324 cumulation of melanin pigment, and the mononucle- 18.1.3.2 Causative Allergens . 325 ar cell infiltrate in the upper dermis are very similar 18.1.3.3 Treatment . 326 to the histopathological manifestations of lichen pla- 18.1.4 Purpuric Dermatitis . 328 nus. However, compared with typical lichen planus, 18.1.5 “Dirty Neck” of Atopic Eczema . 329 hyperkeratosis is usually milder, hypergranulosis 18.2 Depigmentation from Contact and saw-tooth-shape acanthosis are lacking, hyaline with Chemicals . 330 bodies are hardly seen, and the band-like massive in- 18.2.1 Mechanism of Leukoderma filtration with lymphocytes and histiocytes is lack- due to Chemicals . 330 ing. 18.2.2 Contact Leukoderma Caused Mainly by Contact Sensitization . -

Albinism: Modern Molecular Diagnosis
British Journal of Ophthalmology 1998;82:189–195 189 Br J Ophthalmol: first published as 10.1136/bjo.82.2.189 on 1 February 1998. Downloaded from PERSPECTIVE Albinism: modern molecular diagnosis Susan M Carden, Raymond E Boissy, Pamela J Schoettker, William V Good Albinism is no longer a clinical diagnosis. The past cytes and into which melanin is confined. In the skin, the classification of albinism was predicated on phenotypic melanosome is later transferred from the melanocyte to the expression, but now molecular biology has defined the surrounding keratinocytes. The melanosome precursor condition more accurately. With recent advances in arises from the smooth endoplasmic reticulum. Tyrosinase molecular research, it is possible to diagnose many of the and other enzymes regulating melanin synthesis are various albinism conditions on the basis of genetic produced in the rough endoplasmic reticulum, matured in causation. This article seeks to review the current state of the Golgi apparatus, and translocated to the melanosome knowledge of albinism and associated disorders of hypo- where melanin biosynthesis occurs. pigmentation. Tyrosinase is a copper containing, monophenol, mono- The term albinism (L albus, white) encompasses geneti- oxygenase enzyme that has long been known to have a cally determined diseases that involve a disorder of the critical role in melanogenesis.5 It catalyses three reactions melanin system. Each condition of albinism is due to a in the melanin pathway. The rate limiting step is the genetic mutation on a diVerent chromosome. The cutane- hydroxylation of tyrosine into dihydroxyphenylalanine ous hypopigmentation in albinism ranges from complete (DOPA) by tyrosinase, but tyrosinase does not act alone. -

Characteristics and Clinical Manifestations of Pigmented Purpuric Dermatosis
DH Kim, et al Ann Dermatol Vol. 27, No. 4, 2015 http://dx.doi.org/10.5021/ad.2015.27.4.404 ORIGINAL ARTICLE Characteristics and Clinical Manifestations of Pigmented Purpuric Dermatosis Dai Hyun Kim, Soo Hong Seo, Hyo Hyun Ahn, Young Chul Kye, Jae Eun Choi Department of Dermatology, Korea University College of Medicine, Seoul, Korea Background: Pigmented purpuric dermatoses (PPD) are a sults were grossly consistent with the existing literature, ex- spectrum of disorders characterized by a distinct purpuric cluding several findings. Although a possible relationship rash. Although PPD can be easily diagnosed, the disease en- between PPD and cardiovascular disease or cardiovascular tity remains an enigma and a therapeutic challenge. medication was proposed at the beginning of the study, no Objective: The purpose of this study was to investigate the statistically significant correlations were found according to characteristics and clinical manifestations of PPD and to elu- the specific clinical types and treatment responses (p> cidate the relationship between assumed etiologic factors 0.05). (Ann Dermatol 27(4) 404∼410, 2015) and the clinical manifestations of PPD and treatment responses. Methods: Retrograde analyses were performed to -Keywords- identify appropriate PPD patients who visited Korea Classification, Etiology, Pigmented purpuric dermatoses, University Medical Center Anam Hospital from 2002 to Therapy 2012. Results: Information on 113 patients with PPD was an- alyzed, and 38 subjects with skin biopsy were included for this study. Schamberg's disease was the most frequent clin- INTRODUCTION ical type (60.5%). Concomitant diseases included hyper- tension (15.8%), diabetes (10.5%), and others. Associated Pigmented purpuric dermatosis (PPD) is a general term medication histories included statins (13.2%), beta blockers used to describe a group of chronic and relapsing cuta- (10.5%), and others. -

Ocular Colobomaâ
Eye (2021) 35:2086–2109 https://doi.org/10.1038/s41433-021-01501-5 REVIEW ARTICLE Ocular coloboma—a comprehensive review for the clinician 1,2,3 4 5 5 6 1,2,3,7 Gopal Lingam ● Alok C. Sen ● Vijaya Lingam ● Muna Bhende ● Tapas Ranjan Padhi ● Su Xinyi Received: 7 November 2020 / Revised: 9 February 2021 / Accepted: 1 March 2021 / Published online: 21 March 2021 © The Author(s) 2021. This article is published with open access Abstract Typical ocular coloboma is caused by defective closure of the embryonal fissure. The occurrence of coloboma can be sporadic, hereditary (known or unknown gene defects) or associated with chromosomal abnormalities. Ocular colobomata are more often associated with systemic abnormalities when caused by chromosomal abnormalities. The ocular manifestations vary widely. At one extreme, the eye is hardly recognisable and non-functional—having been compressed by an orbital cyst, while at the other, one finds minimalistic involvement that hardly affects the structure and function of the eye. In the fundus, the variability involves the size of the coloboma (anteroposterior and transverse extent) and the involvement of the optic disc and fovea. The visual acuity is affected when coloboma involves disc and fovea, or is complicated by occurrence of retinal detachment, choroidal neovascular membrane, cataract, amblyopia due to uncorrected refractive errors, etc. While the basic birth anomaly cannot be corrected, most of the complications listed above are correctable to a great 1234567890();,: 1234567890();,: extent. Current day surgical management of coloboma-related retinal detachments has evolved to yield consistently good results. Cataract surgery in these eyes can pose a challenge due to a combination of microphthalmos and relatively hard lenses, resulting in increased risk of intra-operative complications.