Review an Overview of the Pale and Clear Cells of the Nipple Epidermis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paraneoplastic Syndrome Presenting As Giant Porokeratosis in a Patient with Nasopharyngeal Cancer
Paraneoplastic Syndrome Presenting As Giant Porokeratosis in A Patient with Nasopharyngeal Cancer Fitri Azizah, Sonia Hanifati, Sri Adi Sularsito, Lili Legiawati, Shannaz Nadia Yusharyahya, Rahadi Rihatmadja Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo National General Hospital Keywords: porokeratosis, giant porokeratosis, paraneoplastic syndrome, nasopharyngeal Abstract: Giant porokeratosis is a rare condition in which the hyperkeratotic plaques of porokeratosis reach up to 20 cm in diameter. Porokeratosis is characterized clinically by hyperkeratotic papules or plaques with a thread-like elevated border. Although rare, porokeratosis has been reported in conjunction with malignancies suggesting a paraneoplastic nature. Associated malignancies reported were hematopoietic, hepatocellular, and cholangiocarcinoma. We report a case of giant porokeratosis in a patient with nasopharyngeal cancer responding to removal of the primary cancer by chemoradiotherapy. 1 INTRODUCTION regress completely after the treatment of malignancy, suggestive of paraneoplastic syndrome. Porokeratosis is a chronic progressive disorder of keratinization, characterized by hyperkeratotic papules or plaques surrounded by a thread-like 2 CASE elevated border corresponds to a typical histologic hallmark, the cornoid lamella . O regan, 2012) There Mr. SS, 68-year-old, was referred for evaluation of are at least six clinical variants of porokeratosis pruritic, slightly erythematous plaques with raised, recognized with known genetic disorder.1 Some hyperpigmented border of one and a half year clinical variant of porokeratosis has been reported in duration on the extensor surface of both legs. The the setting of immunosuppressive conditions, organ lesions shown minimal response to potent topical transplantation, use of systemic corticosteroids, and corticosteroids and phototherapy given during the infections, suggesting that impaired immunity may last 8 months in another hospital. -

Features of Reactive White Lesions of the Oral Mucosa
Head and Neck Pathology (2019) 13:16–24 https://doi.org/10.1007/s12105-018-0986-3 SPECIAL ISSUE: COLORS AND TEXTURES, A REVIEW OF ORAL MUCOSAL ENTITIES Frictional Keratosis, Contact Keratosis and Smokeless Tobacco Keratosis: Features of Reactive White Lesions of the Oral Mucosa Susan Müller1 Received: 21 September 2018 / Accepted: 2 November 2018 / Published online: 22 January 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract White lesions of the oral cavity are quite common and can have a variety of etiologies, both benign and malignant. Although the vast majority of publications focus on leukoplakia and other potentially malignant lesions, most oral lesions that appear white are benign. This review will focus exclusively on reactive white oral lesions. Included in the discussion are frictional keratoses, irritant contact stomatitis, and smokeless tobacco keratoses. Leukoedema and hereditary genodermatoses that may enter in the clinical differential diagnoses of frictional keratoses including white sponge nevus and hereditary benign intraepithelial dyskeratosis will be reviewed. Many products can result in contact stomatitis. Dentrifice-related stomatitis, contact reactions to amalgam and cinnamon can cause keratotic lesions. Each of these lesions have microscopic findings that can assist in patient management. Keywords Leukoplakia · Frictional keratosis · Smokeless tobacco keratosis · Stomatitis · Leukoedema · Cinnamon Introduction white lesions including infective and non-infective causes will be discussed -

An Update on the Biology and Management of Dyskeratosis Congenita and Related Telomere Biology Disorders
Expert Review of Hematology ISSN: 1747-4086 (Print) 1747-4094 (Online) Journal homepage: https://www.tandfonline.com/loi/ierr20 An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch & Sharon A. Savage To cite this article: Marena R. Niewisch & Sharon A. Savage (2019) An update on the biology and management of dyskeratosis congenita and related telomere biology disorders, Expert Review of Hematology, 12:12, 1037-1052, DOI: 10.1080/17474086.2019.1662720 To link to this article: https://doi.org/10.1080/17474086.2019.1662720 Accepted author version posted online: 03 Sep 2019. Published online: 10 Sep 2019. Submit your article to this journal Article views: 146 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ierr20 EXPERT REVIEW OF HEMATOLOGY 2019, VOL. 12, NO. 12, 1037–1052 https://doi.org/10.1080/17474086.2019.1662720 REVIEW An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch and Sharon A. Savage Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA ABSTRACT ARTICLE HISTORY Introduction: Telomere biology disorders (TBDs) encompass a group of illnesses caused by germline Received 14 June 2019 mutations in genes regulating telomere maintenance, resulting in very short telomeres. Possible TBD Accepted 29 August 2019 manifestations range from complex multisystem disorders with onset in childhood such as dyskeratosis KEYWORDS congenita (DC), Hoyeraal-Hreidarsson syndrome, Revesz syndrome and Coats plus to adults presenting Telomere; dyskeratosis with one or two DC-related features. -

Incidental Focal Acantholytic Dyskeratosis in the Setting of Rosacea
Letter to the Editor http://dx.doi.org/10.5021/ad.2013.25.4.518 Incidental Focal Acantholytic Dyskeratosis in the Setting of Rosacea Sang-Yeon Park, Hae Jin Lee, Jae Yong Shin, Sung Ku Ahn Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea Dear Editor: direct immunofluorescence (Fig. 2). In the serial sections, Focal acantholytic dyskeratosis (FAD) was first described we observe the same findings. Differential diagnosis for by Ackerman1 in 1972 as a distinct histopathological pat- the possibility of polymorphous light eruption, systemic tern associated with various cutaneous conditions, and lupus erythematosus, contact dermatitis, and dermatitis with classic histopathological findings including supra- artefacta should be considered. But given these clinical basal clefting, hyperkeratosis and parakeratosis, and the and histopathological features, a diagnosis of rosacea with presence of acantholytic and dyskeratotic cells at the FAD was reached. The patient was then admitted to epidermis. While FAD can be observed in many various hospital for treatment with doxycycline 100 mg and cutaneous lesions including benign and/or malignant antihistamines. After one week, the lesions had remarka- epithelial lesions, fibrohistiocytic lesions, inflammatory bly improved. The patient was then discharged, and lesions, melanocytic and/or follicular lesions2-4. These continued on the same therapeutic regimen for an histopathological findings may also extend into the additional month, bythe time all lesions were nearly surrounding tissues, which often appear to be clinically resolved. normal. A 42-year-old woman was presented to our To date, the etiology of FAD has been attributed to department with multiple erythematous pruritic papules numerous sources including hormones, viral infection, and tiny vesicles on her face. -

Boards Fodder Disorders of Dyschromia (Hypo- and Hyperpigmentation) by Parin Pearl Rimtepathip, MD, and Janna Mieko Vassantachart, MD
boards fodder Disorders of dyschromia (hypo- and hyperpigmentation) by Parin Pearl Rimtepathip, MD, and Janna Mieko Vassantachart, MD Genetic conditions Gene Disorder Pathophysiology Clinical Features (Unique Features) Mutation Dyskeratosis XLR (MC): Reduced telomerase activ- Male > Female. Bone marrow failure up to Congenita DKC 1 ity and abnormally short- 90% (increase risk of hematopoietic malig- (Zinsser-Engman- ened telomeres chro- nancies) + triad of abnormal skin pigmenta- Cole syndrome) AD: TERT, mosomal instability/cellu- tion (poikilodermatous patches of face/neck/ TERC lar replication dysfunction upper torso), onychodystrophy, premalignant oral leukoplakia (vs benign oral leukoplakia in Pachyonychia Congenita type I) Dyschromatosis AD: ADAR Heterozygous mutations in Presents by 6-years-old with hyper/hypopig- Symmetrica (SDAR the gene encodes an RNA mented macules restricted to sun-exposed Hereditaria gene) specific adenosine deami- skin on the dorsal aspects of bilateral (Reticulate nase extremities and face Acropigmentation of Dohi) Naegeli- AD: Location of expression of Allelic to DPR. Brown gray reticulated hyper- Franceschetti- Keratin keratin 14 - Basal kerati- pigmentation typically localized to abdomen, Jadassohn 14 nocytes develops around age 2 and improves after Syndrome (NFJS) puberty. Other findings: PPK + adermato- glyphia (no finger prints) + dental anomalies including early loss of teeth (not seen in DPR) + hypohidrosis + onychodystrophy Dermatopathia AD: Location of expression of Allelic to NFJS. Unique features: diffuse non- Pigmentosa Keratin keratin 14 - Basal kerati- scarring alopecia (not seen in NFJS) + ony- Reticularis (DPR) 14 nocytes chodystrophy + adermatoglyphia + persistent reticulated hyperpigmentation of torso and proximal UE + No dental anomalies Dyschromatosis AD/AR: Mutation in ATP bind- Japanese. Torso predominant with mottled Universalis ABCB6 ing cassette subfamily B, appearance, nail dystrophy, and pterygium. -

Review of the Literature
Review Clinical and Histopathological Features and Potential Pathological Mechanisms of Skin Lesions in COVID-19: Review of the Literature Gürkan Kaya 1,*, Aysin Kaya 2 and Jean-Hilaire Saurat 2 1 Departments of Dermatology and Clinical Pathology, University Hospital of Geneva, 1205 Geneva, Switzerland 2 Department of Clinical Pharmacology and Toxicology, University of Geneva, 1205 Geneva, Switzerland; [email protected] (A.K.); [email protected] (J.-H.S.) * Correspondence: [email protected] Received: 24 June 2020; Accepted: 29 June 2020; Published: 30 June 2020 Abstract: In recent weeks, several reports have emerged of skin lesions with different clinical presentations in COVID-19 cases. All dermatologists should be aware of these cutaneous lesions, which may be early clinical symptoms of infection. We reviewed the literature on cutaneous manifestations in the PubMed database from December 2019 and June 2020. From the cases described as case reports or series in 57 recent articles, it appears that skin lesions (i) are highly varied, (ii) may not be related to the severity of the condition and (iii) resolve spontaneously in a few days. The frequency of these lesions in COVID-19 patients varies between 1.8% and 20.4%. The major clinical forms described were maculopapular eruptions, acral areas of erythema with vesicles or pustules (pseudochilblain), urticarial lesions, other vesicular eruptions and livedo or necrosis. The lesions were mainly localized in the trunk and extremities. The majority of patients were male, aged between 4.5 and 89 years. A minority of the patients were children presenting with acral, chilblain-like lesions, papulo-vesicular eruptions or Kawasaki disease-like pediatric inflammatory multisystem syndrome. -

Boards' Fodder
boards’ fodder Disorders of Hyperpigmentation by Sarah Brooks, M.D. GENETIC Gene Pathophysiology Clinical Features Dyskeratosis congenita XLR: Mutation in dyskerin protein Lacy reticulated hyperpigmentation on the neck, upper arms, DKC1 which interacts with telom- upper chest. Pterygium, leukoplakia, pancytopenia, mucosal gene erase, or mutation in telom- squamous cell carcinoma, leukemia AD: hTR, erase subunits hTERT Naegeli–Franceschetti– AD: Mutation in non-helical head Periocular, perioral, abdominal gray-brown reticulate hyperpig- Jadassohn KRT14 domain of keratin 14 lead- mentation. Fades after puberty. Decreased sweat glands w/ heat ing to early termination of intolerance, dental anomalies, absent dermatoglyphics. translation Dermatopathia (pos- Mutation in non-helical head Triad: reticulate hyperpigmentation of trunk and proximal extremi- pigmentosa reticularis sible) AD: domain of keratin 14. ties, non-scarring alopecia, and onychodystrophy. Does not fade KRT14 after puberty. X-Linked Reticulate X-Linked Unknown Male: generalized hyperpigmentation, onset 4 mo to 5 yrs. pigmentary disorder Severe systemic manifestations (recurrent pneumonia, COPD , early death). Blonde unruly hair with a frontal upsweep, +/- low intelligence. Female: skin-limited manifestations with lacy or reticulated hyperpigmentation w/in lines of Blaschko. Dowling-Degos disease AD: KRT5 Loss of function mutation Reticulate hyperpigmentation, beginning in axillae and groin and (DDD) (possible role of keratin 5 spreading to other body folds. Comedone-like lesions on the in melanosome uptake & back or neck, pitted perioral or facial scars. organelle transport) Galli-Galli Disease AD Acantholytic variant of DDD. Same as DDD Reticulate AD, possi- May be on a spectrum with Atrophic lentigo-like reticulated hyperpigmented macules on the acropigmentation of bly KRT5 DDD. -
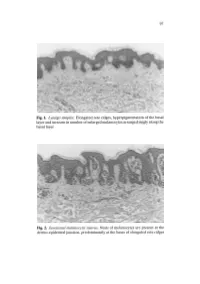
Fig. 1. Lentigo Simplex. Elongated Rete Ridges, Hyperpigmentation of The
97 Fig. 1. Lentigo simplex. Elongated rete ridges, hyperpigmentation of the basal layer and increase in number of enlarged melanocytes arranged singly along the basal layer Fig. 2. Junctional melanocytic naevus. Nests of melanocytes are present at the dermo-epidermal junction, predominantly at the bases of elongated rete ridges 98 a b Fig.3a,b. Compound melanocytic naevus. a Nests of melanocytes in the dermis as well as the derma-epidermal junction, smaller and more diffusely arranged in the deeper dermis. b The nests of melanocytes include multinucleated giant cells 99 Fig. 4. Dermal melanocytic naevus. Entirely intradermal tumour in a nested pattern in the superficial layers, composed of small naevoid melanocytes in the deep dermis Fig. 5. Dermal melanocytic naevus. The deeper layers of this dermal melanocytic naevus include neuroid structures similar to Wagner-Meissner corpuscles (neuroid dermal melanocytic naevus) 100 Fig. 6. Balloon cell naevus. The tumour is composed mainly of melanocytes with abundant clear cytoplasm and central nuclei Fig. 7. Halo naevus. Compound naevus with a dense lymphocytic infiltrate between the naevoid melanocytes of the dermal component Fig. 8. Combined intradermal naevus and blue naevus. Nests of epithelioid naevus cells in the dermis (right) and heavily pigmented spindle and dendritic cells arranged between collagen bundles in the adjacent stroma (left) Fig. 9a. Deep penetrating naevus. A wedge-shaped lesion composed of irregu lar collections of variably pigmented cells extending into the deep dermis. 102 Fig. 9b. Deep penetrating naevus. In the deep dermis the lesion includes large cells with nuclear pseudo-inclusions, smaller naevoid melanocytes and groups of melanophages in the intervening stroma Fig. -
Polarization Optical Studies of Hyperkeratosis, Parakeratosis and Dyskeratosis* Daphne Anderson Roe, M.D., M.R.C.P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector POLARIZATION OPTICAL STUDIES OF HYPERKERATOSIS, PARAKERATOSIS AND DYSKERATOSIS* DAPHNE ANDERSON ROE, M.D., M.R.C.P. Previous polarization optical studies of adultlife, the epidermis reaches its final state; at this and embryonic human epidermis have beenstage the eornified layer shows intense double devoted to the elucidation of the morphology ofrefraction. It is of interest that in the chick its fibrous structure. In unstained verticalembryo, the long axis of the cells of the periderm sections of human abdominal skin, the mostand of the embryonic horny layer is parallel to intense double refraction occurs in the stratumthe skin surface and the early keratin fibres are corneum and stratum lucidum. In these layersalso oriented in the same plane. birefringent material is oriented parallel to the There is only one polarization optical study of surface plane; below this region the birefringentpathologic epidermis. Nieuwmeijer examined the fibres are oriented perpendicularly to the surfacetonofibrils in bullous dermatoses by this method (1). and found differences between the tonofibrillar Similar observations have been made by othersystems in pemphigus and dermatitis herpeti- authors (2, 3), among them Mercer, who hasformis (6). In pemphigus, in the areas of acan- compared the appearance of the epidermis undertholysis, the tonofibrils were greatly decreased, the polarization and electron microscopes. Hewhereas in dermatitis herpetiformis the tonofibrils noted that the fibrils (tonofibrils) in the lowerwere pushed aside by the fluid in the bullae. layers of the epidermis were weakly birefringent. Recently, I used polarization optical methods At the level of the appearance of keratohyalinto investigate the anomaly of keratin formation there was a sudden rise in birefringenee associatedin psoriasis (7). -

Pagetoid Dyskeratosis of the Male Genitalia: Case Report and Review
Open Access Case Report DOI: 10.7759/cureus.2727 Pagetoid Dyskeratosis of the Male Genitalia: Case Report and Review Tyler Werbel 1 , Philip R. Cohen 2 1. School of Medicine, University of California, San Diego, San Diego, USA 2. Dermatologist, San Diego Family Dermatology, National City, USA Corresponding author: Tyler Werbel, [email protected] Abstract Pagetoid dyskeratosis is a benign incidental pathologic finding that has been reported in many distinct skin lesions on various locations of the body. A man who had pagetoid dyskeratosis within lesions of the penile shaft is described and similar cases of pagetoid dyskeratosis in lesions of the male genitalia are reviewed. The patient was a 26-year-old healthy man who developed several asymptomatic penile papules that were refractory to topical imiquimod 5% cream and cryotherapy. Snip biopsies were performed and microscopic examination revealed pagetoid dyskeratosis. PubMed was searched for the following terms: cell, clear, dyskeratosis, genitalia, pagetoid, penile, penis, prepuce, scrotum, and shaft. The papers containing these terms and their references were reviewed. Pagetoid dyskeratosis has been observed in lesions on the prepuce and scrotum; this case report now expands the distribution of this finding to the penile shaft. Clinicians and pathologists should be aware of this intriguing potential incidental finding within skin lesions of the male genitalia. Categories: Dermatology, Pathology, Urology Keywords: cell, clear, dyskeratosis, genitalia, pagetoid, penile, penis, prepuce, scrotum, shaft Introduction Pagetoid dyskeratosis is a benign incidental pathologic feature. It has been observed in several skin lesions [1]. Herein, a man with penile papules that demonstrated pagetoid dyskeratosis is described, and patients with pagetoid dyskeratosis of the male genitalia are summarized. -

The Best Diagnosis Is
Dermatopathology Diagnosis Verruciform Xanthoma The best diagnosis is: H&E, original magnification 100. a. epidermolytic acanthoma b. myrmecia CUTISc. verruca vulgaris d. verruciform xanthoma Do Note. wartyCopy dyskeratoma H&E, original magnification 200 (original magnification 400 [inset in bottom right corner]). PLEASE TURN TO PAGE 285 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Maria R. Robinson, MD; Shane A. Meehan, MD From the Ronald O. Perelman Department of Dermatology, New York University, New York. The authors report no conflict of interest. Correspondence: Maria R. Robinson, MD, Ronald O. Perelman Department of Dermatology, New York University, 530 First Ave, Ste 7J, New York, NY 10016 ([email protected]). 272 CUTIS® WWW.CUTIS.COM Copyright Cutis 2013. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Verruciform Xanthoma erruciform xanthomas typically present as the presence of koilocytes, coarse hypergranulosis, asymptomatic, flat, solitary papules or plaques papillomatosis, and rete ridges that curve inward Von the oral mucosa but also can occur on (Figure 3). Myrmecia are predisposed to involvement the genital mucosa and other cutaneous sites. On of palmoplantar skin and are associated with Human low-power magnification, they have a verruca papillomavirus 1. Myrmecia characteristically have vulgaris–like appearance due to characteristic acan- larger eosinophilic keratohyalin granules within the thosis, papillomatosis, and hyperkeratosis (Figure 1). upper spinous layers and display a more endophytic Additionally, there often are numerous neutrophils growth pattern (Figure 4). Warty dyskeratomas also in the upper layers of the epidermis with overlying have an endophytic growth pattern, but they dem- parakeratosis. -

37 Histopathology of Irritant Contact Dermatitis
345 37 Histopathology of Irritant Contact Dermatitis Carolyn M. Willis Contents the skin [1], and with a histopathology largely indis- tinguishable from that of the majority of chronic in- 37.1 Introduction . 345 flammatory dermatoses. 37.2 Acute Irritant Contact Dermatitis . 346 When considering the histopathology of ICD, it is 37.2.1 Epidermal Features . 346 important to bear in mind that all of the following pa- 37.2.2 Dermal Features . 348 rameters will influence the histopathological changes 37.2.3 Leukocyte Infiltration . 349 observed under the light microscope: 37.3 Chronic Irritant Contact Dermatitis . 350 37.3.1 Epidermal Features . 350 37.3.2 Dermal Features . 350 1. Chemical nature and concentration of irritant References . 350 chemical In addition to the physicochemical properties of an irritant, which have a direct bearing on the nature of the cellular damage inflicted, concentration effects are also profound. At sufficiently high concentration, 37.1 Introduction many irritants will cause overt tissue necrosis. Lower concentrations produce more subtle changes, partic- Irritant contact dermatitis is a heterogeneous inflam- ularly in the epidermis. matory condition, both clinically and histopathologi- cally. Arising primarily from contact with chemicals, 2. Mode and duration of exposure the inflammation may be acute or chronic in nature, The circumstances of irritant exposure, such as single, depending upon the irritation potential of the sub- occlusive patch testing or repetitive open testing, and stance and the circumstances of exposure. Chemicals the length of time the chemical is in contact with the such as acids, alkalis, and detergents will, at high con- skin, will all influence the severity and nature of re- centration, cause sufficient damage to the skin to in- sponse, and hence the histological picture.