WSC 13-14 Conf 13 Layout
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Pathomorpholog.Pdf
Ukrаiniаn Medicаl Stomаtologicаl Аcаdemy THE DEPАRTАMENT OF PАTHOLOGICАL АNАTOMY WITH SECTIONSL COURSE MАNUАL for the foreign students GENERАL PАTHOMORPHOLOGY Poltаvа-2020 УДК:616-091(075.8) ББК:52.5я73 COMPILERS: PROFESSOR I. STАRCHENKO ASSOCIATIVE PROFESSOR O. PRYLUTSKYI АSSISTАNT A. ZADVORNOVA ASSISTANT D. NIKOLENKO Рекомендовано Вченою радою Української медичної стоматологічної академії як навчальний посібник для іноземних студентів – здобувачів вищої освіти ступеня магістра, які навчаються за спеціальністю 221 «Стоматологія» у закладах вищої освіти МОЗ України (протокол №8 від 11.03.2020р) Reviewers Romanuk A. - MD, Professor, Head of the Department of Pathological Anatomy, Sumy State University. Sitnikova V. - MD, Professor of Department of Normal and Pathological Clinical Anatomy Odessa National Medical University. Yeroshenko G. - MD, Professor, Department of Histology, Cytology and Embryology Ukrainian Medical Dental Academy. A teaching manual in English, developed at the Department of Pathological Anatomy with a section course UMSA by Professor Starchenko II, Associative Professor Prylutsky OK, Assistant Zadvornova AP, Assistant Nikolenko DE. The manual presents the content and basic questions of the topic, practical skills in sufficient volume for each class to be mastered by students, algorithms for describing macro- and micropreparations, situational tasks. The formulation of tests, their number and variable level of difficulty, sufficient volume for each topic allows to recommend them as preparation for students to take the licensed integrated exam "STEP-1". 2 Contents p. 1 Introduction to pathomorphology. Subject matter and tasks of 5 pathomorphology. Main stages of development of pathomorphology. Methods of pathanatomical diagnostics. Methods of pathomorphological research. 2 Morphological changes of cells as response to stressor and toxic damage 8 (parenchimatouse / intracellular dystrophies). -

'Spongiosis' Dermatitis With
Spongiosis Spongiosis and Spongiotic • What is ‘spongiosis’? – Intra-epidermal and Dermatitis intercellular edema • Widening of intercellular spaces between keratinocytes • Elongation of G.Peter Sarantopoulos, MD intercellular bridges UCLA Medical Center Spongiosis vs. Spongiotic Spongiosis Dermatitis • ‘Spongiosis’ as a histologic concept (not a • Not everything ‘spongiotic’ is a diagnosis!) spongiotic dermatitis – Intra-epidermal edema accompanies many (if not all) inflammatory skin diseases to some degree • So-called ‘patterns of spongiosis’ • Important to distinguish spongiosis as… – Neutrophilic – The predominant histologic finding – Eosinophilic – A non-specific feature of other inflammatory – Follicular dermatoses (e.g. lichenoid/interface, vasculopathic, – Miliarial psoriasiform, etc) – Sometimes, there is overlap Dermatitis with ‘Spongiosis’ Dermatitis with ‘Spongiosis’ * Neutrophilic: Eosinophilic: Miliarial: Neutrophilic: Eosinophilic: Miliarial: Pustular psoriasis Pemphigus (precursor) M. Crystallina Pustular psoriasis Pemphigus (precursor) M. Crystallina Reiter’s syndrome Pemphigus vegetans M. Rubra Reiter’s syndrome Pemphigus vegetans M. Rubra IgA Pemphigus Bullous pemphigoid M. profunda IgA Pemphigus Bullous pemphigoid M. profunda Pemphigus herpetiformis Cicatricial pemphigoid Pemphigus herpetiformis Cicatricial pemphigoid Infantile acropustulosis Pemphigoid (herpes) Infantile acropustulosis Pemphigoid (herpes) AGEP gestationis Follicular: AGEP gestationis Follicular: Palmoplantar pustulosis Idiopathic eosinophilic Infundibulofolliculitis -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Lumps & Bumps: Approach to Common Dermatologic Neoplasms
Case-Based Approach to Common Dermatologic Neoplasms Patrick Retterbush, MD, FAAD Mohs Surgery & Dermatologic Oncology Associate Member of the American College of Mohs Surgery Private Practice: Lockman Dermatology January 27th 2018 Disclosure of Relevant Financial Relationships • I do not have any relevant financial relationships, commercial interests, and/or conflicts of interest regarding the content of this presentation. Goals/Objectives • Recognize common benign growths • Recognize common malignant growths • Useful clues & examination for evaluating melanocytic nevi and when to be concerned for melanoma/atypical moles • How to perform a basic skin biopsy and which method/type to choose • Basic treatment/when to refer Key Questions & Physical Examination Findings for a Growth History Physical Examination • How long has the lesion been • Describing a growth present? – flat or raised? • flat – macule (<1cm) or patch (>1cm) – years, months, weeks • raised – papule (<1cm) or plaque (>1cm) – nodule if deep (majority of lesion in • Has it changed? dermis/SQ) – Size – secondary descriptive features • scaly (hyperkeratosis, retention of strateum – Shape corneum) – Color • crusty (dried serum, blood, or pus on surface) • eroded or ulcerated (partial vs. full thickness – Symptoms – pain, bleeding, itch? epidermal loss) – Over what time frame? • color (skin colored, red, pigmented, pearly) • feel (hard or soft, mobile or fixed) • PMH: • size: i.e. 6 x 4mm – prior skin cancers • Look at the rest of the skin/region of skin • SCC/BCCs vs. melanoma -

SKIN VERSUS PEMPHIGUS FOLIACEUS and the AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital
VETERINARY NURSING EDUCATION SKIN VERSUS PEMPHIGUS FOLIACEUS AND THE AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital This program was reviewed and approved by the AAVSB Learning Objective: After reading this article, the participant will be able to dis- RACE program for 1 hour of continuing education in jurisdictions which recognize AAVSB RACE approval. cuss and compare autoimmune diseases that have dermatological afects, includ- Please contact the AAVSB RACE program if you have any ing Pemphigus Foliaceus (PF), Pemphigus Erythematosus (PE), Discoid Lupus comments/concerns regarding this program’s validity or relevancy to the veterinary profession. Erythematosus (DLE), Systemic Lupus Erythematosus (SLE). In addition, the reader will become familiar with diagnostic and treatment techniques. FUNCTION OF THE SKIN Te skin is the largest organ of the body. Along with sensory function, it provides a barrier between the inside and outside world. Te epidermis is composed of the following fve layers: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. Te stratum lucidum is found only on the nasal planum and footpads. When the cells of the epidermis are disrupted by systemic disease, the barrier is also disrupted. Clinical signs of skin disease will bring the patient into the veterinarian’s ofce for diagnosis. 32 THE NAVTA JOURNAL | NAVTA.NET VETERINARY NURSING EDUCATION THE PEMPHIGUS COMPLEX article.3 Histologically it shares characteris- Pemphigus Foliaceus tics of both PF and DLE.1 This classifcation PF is an immune mediated pustular disor- is still considered controversial and PE may der included in a group of diseases known just be a localized variant of PF.1 as the pemphigus complex. -

Wednesday Slide Conference 2008-2009
PROCEEDINGS DEPARTMENT OF VETERINARY PATHOLOGY WEDNESDAY SLIDE CONFERENCE 2008-2009 ARMED FORCES INSTITUTE OF PATHOLOGY WASHINGTON, D.C. 20306-6000 2009 ML2009 Armed Forces Institute of Pathology Department of Veterinary Pathology WEDNESDAY SLIDE CONFERENCE 2008-2009 100 Cases 100 Histopathology Slides 249 Images PROCEEDINGS PREPARED BY: Todd Bell, DVM Chief Editor: Todd O. Johnson, DVM, Diplomate ACVP Copy Editor: Sean Hahn Layout and Copy Editor: Fran Card WSC Online Management and Design Scott Shaffer ARMED FORCES INSTITUTE OF PATHOLOGY Washington, D.C. 20306-6000 2009 ML2009 i PREFACE The Armed Forces Institute of Pathology, Department of Veterinary Pathology has conducted a weekly slide conference during the resident training year since 12 November 1953. This ever- changing educational endeavor has evolved into the annual Wednesday Slide Conference program in which cases are presented on 25 Wednesdays throughout the academic year and distributed to 135 contributing military and civilian institutions from around the world. Many of these institutions provide structured veterinary pathology resident training programs. During the course of the training year, histopathology slides, digital images, and histories from selected cases are distributed to the participating institutions and to the Department of Veterinary Pathology at the AFIP. Following the conferences, the case diagnoses, comments, and reference listings are posted online to all participants. This study set has been assembled in an effort to make Wednesday Slide Conference materials available to a wider circle of interested pathologists and scientists, and to further the education of veterinary pathologists and residents-in-training. The number of histopathology slides that can be reproduced from smaller lesions requires us to limit the number of participating institutions. -
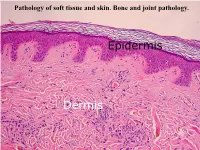
Pathology of Soft Tissue and Skin. Bone and Joint Pathology. Pathology of Soft Tissue and Skin
Pathology of soft tissue and skin. Bone and joint pathology. Pathology of soft tissue and skin. Bone and joint pathology. I.Microspecimens: № 188. Capillary hemangioma. (H.E. stain). Indications: 1. Epidermis. 2. Dermis 3. Spindle cells arranged compactly with spaces containing blood. 4. Reduced connective stroma. In the microspecimen is presented a well-defined subepidermal tumoral node, consisting of proliferating capillary blood vessels, poor loose stroma; epidermis with normal histological structure. Hemangioma is a benign tumor of vascular origin, histological variants are capillary, venous and cavernous hemangioma. It is located mainly in the skin, the mucosa of the gastrointestinal tract, the liver. Capillary hemangioma is the most common benign tumor in children and has a disembryoplazic character, being interpreted as a hamartoma - a tumor from the embryonic tissues. Macroscopically it has the appearance of a red-purple node or plaque. Cutaneous hemangiomas can be complicated by exulceration, bleeding, the association of secondary infection. 3 2 4 1 № 188. Capillary hemangioma. (H.E. stain). № 43. Fibrosarcoma. (H.E. stain). Indications: 1.Epidermis. 2.Dermis. 3.Atypical tumor cells (fibroblast-like). 4.Bundles of collagen fibers. In the skin, under the epidermis there is a rich cellular tumoral node, consisting of predominantly spindle-shaped cells, of the fibroblasts type, arranged in bundles, which intersect in different directions, the tumor has no precise limits, many mitoses, giant cells, foci of necrosis, hemorrhage, stroma is poor. Fibrosarcoma is a malignant tumor, which derives from fibroblasts, may have different degrees of differentiation. It is found in adults between the ages of 40 and 70, located more frequently in the deep tissues of the hip, knee, in the retroperitoneal area. -

Tern, It Was Felt of Use to Record the Experience in This Matter
PERSISTENT "INSECT BITES" (DERMAL EOSINOPHILIC GRANU- LOMAS) SIMULATING LYMPHOBLASTOMAS, HISTIOCYTOSES, AND SQUAMOUS CELL CARCINOMAS * ARrHUR C. ALLEN, MD. t (From the Army instute of Pathology, Washingtox 25, D.C.) In 1942, opportunity was afforded at the Army Institute of Pathol- ogy to review the histologic slides of a lesion said to have been pro- duced by a tick bite. The microscopic sections seemed at the time indistinguishable from mycosis fungoides or Hodgkin's disee, espe- cialy in view of the presence of multiple lesions in the patient. How- ever, following the study of the cutaneous reactions to arthropods (ticks, mosquitoes, and chiggers), it was quickly appreciated that not only were these diagnoses of neoplasia wrong but that the misinterpretation of these reactions was a common and serious error.' The errors in- volved the misconstruction not only of the dermal reaction but also of the epidermal changes. The latter response was confused with squa- mous cell carcinoma; the dermal infitrate was mistaken for mycosis fungoides, Hodgkin's disease, lymphosacoma, giant follicular lympho- blastoma, and Spiegler-Fendt sarcoid. Undoubtedly the principal rea- son for the failure to attnbute these reactions properly to bites of arachnida and insects was referable to the general impression, despite dear-cut cinical histories, that such reactions last only for days, whereas, in truth, they may persist for as long as 2 years. More re- cently, the problem has been further complicated by introduction into the literature of a lesion called "eosinophilic granuloma of skin," an entity of questionable nosologic justification.' Therefore, because of the major importance of establishing a definitive diagnosis and because of the interest in the pathogenesis of a much mimicked histologic pat- tern, it was felt of use to record the experience in this matter. -

Description, Definition and Diagnosis of Common Skin Rashes
Description, Definition and Diagnosis of Common Skin Rashes Daniel Zelac, MD Scripps Clinic Acknowledgements . Conflicts of Interest – None . Many of the photographs and diagrams contained in this talk can be referenced in Clinical Dermatology, 5th Edition By Thomas P. Habif, MD . Please do not further duplicate these images . (referenced in talk as “Habif 5th”) What is a rash? . Definition by Webster’s – an eruption of the body . Definition - The popular term for a group of spots or red, inflamed skin that is usually a symptom of an underlying condition or disorder. Often temporary, a rash is only rarely a sign of a serious problem. The Free Dictionary by Farlex . http://medical-dictionary.thefreedictionary.com/Rashes Can we make the diagnosis based solely on one finding? Finding the clues to diagnosis Lesion Types . Primary Lesion - Typically the earliest representative physical finding related to a disease or a condition . Secondary Lesion – A physical finding that develops during the evolution of a disease or condition and can often be affected by the interaction with the patient or others Distribution . Symmetry . Linear . Sun-exposed . Geographic . Accessible . Serpiginous . Palmar/Plantar . Annular . Inguinal/Intertriginous . Hair-bearing . Mucosal . Dermatome Primary Lesions . Macule - Flat circumscribed skin demonstrating a variation in color from surrounding skin <1cm diameter . Patch – Large macule > 1 cm diameter Primary Lesions . Papule – Solid palpable lesion < 0.5cm diameter . Plaque- a broad papule demonstrating elevation from the surrounding skin >0.5 cm diameter, appear relatively flat with no, or limited deep component . Nodule- a larger palpable solid elevation >0.5 cm diameter, often with a deep component Primary Lesions . -

A Multifaceted Examination of Cutaneous Disease Associated
A Multifaceted Examination of Cutaneous Disease Associated With Oncologic Conditions: Atypical Post- Radiation Vascular Proliferation, Cutaneous Neoplasms in Lynch Syndrome Patients, Non-Melanoma Skin Cancer in Children, and Case Studies of Rare Cutaneous Eruptions The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Zhong, Connie S. 2020. A Multifaceted Examination of Cutaneous Disease Associated With Oncologic Conditions: Atypical Post- Radiation Vascular Proliferation, Cutaneous Neoplasms in Lynch Syndrome Patients, Non-Melanoma Skin Cancer in Children, and Case Studies of Rare Cutaneous Eruptions. Doctoral dissertation, Harvard Medical School. Citable link https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37365212 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! ! ! ! ! "!#$%&'()*+&+,!-.)/'0)&'10!1(!2$&)0+1$3!4'3+)3+!"331*')&+,!5'&6!70*1%18'*! 210,'&'1039!"&:;'*)%!<13&=>),')&'10!?)3*$%)@!<@1%'(+@)&'10A!2$&)0+1$3! B+1;%)3/3!'0!C:0*6!D:0,@1/+!;)&'+0&3A!B10=#+%)01/)!DE'0!2)0*+@!'0!26'%,@+0A! )0,!2)3+!D&$,'+3!1(!>)@+!2$&)0+1$3!-@$;&'103F! ! ! ! ! ! ! ! ! ! ! ! G:! ! 2100'+!DF!H6108! ! ! D$G/'&&+,!'0!<)@&')%!I$%('%%/+0&!1(!&6+!>+J$'@+/+0&3!(1@!&6+!#F4F!4+8@++! 5'&6!K101@3!'0!)!D;+*')%!I'+%,!)&!K)@L)@,!#+,'*)%!D*611%! ! ! ! I+G@$)@:!MNA!ONON! Abstract A wide variety of skin conditions can arise in cancer patients, whether from cancer therapy, underlying genetic syndromes, paraneoplastic processes, or immunosuppression. -

Ministry of Health of Ukraine Ukrainian Medical Stomatolgical Academy
Ministry of Health of Ukraine Ukrainian Medical Stomatolgical Academy Methodical Instructions for independent work of students during the training for the practical studies Academic discipline Surgical stomatology Моdule № 3 The topic of the stadies Epithelial tumors of the soft tissues. Tumor-like № 1 formations of the soft tissues: atheroma, rhinophyma, keratoacanthoma, keratolytic papilloma (skin horn). Clinical manifestations, differential diagnosis and treatment. Course IV Faculty Foreign Students Training, Stomatological Poltava – 2020 1 1. Relevance of the topic: The Skin Cancer Foundation recommends the monthly head-to-toe self examination of the skin, so that one can find any new or changing lesions that might be cancerous or precancerous. Skin cancers found and removed early are almost always curable. Learn about the warnings signs of skin cancer and what to look for during a self examination. If you spot anything suspicious, see a doctor. Performed regularly, self-examination can alert you to changes in your skin and aid in the early detection of skin cancer. It should be done often enough to become a habit, but not so often as to feel like a bother. For most people, once a month is ideal, but ask your doctor if you should do more frequent checks. You may find it helpful to have a doctor do a full-body exam first, to assure you that any existing spots, freckles, or moles are normal or treat any that may not be. After the first few times, self-examination should take no more than 10 minutes – a small investment in what could be a life-saving procedure.