The Interface Reaction Pattern in the Skin: an Integrated Review of Clinical and Pathological Features☆,☆☆ Maria A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Pathomorpholog.Pdf
Ukrаiniаn Medicаl Stomаtologicаl Аcаdemy THE DEPАRTАMENT OF PАTHOLOGICАL АNАTOMY WITH SECTIONSL COURSE MАNUАL for the foreign students GENERАL PАTHOMORPHOLOGY Poltаvа-2020 УДК:616-091(075.8) ББК:52.5я73 COMPILERS: PROFESSOR I. STАRCHENKO ASSOCIATIVE PROFESSOR O. PRYLUTSKYI АSSISTАNT A. ZADVORNOVA ASSISTANT D. NIKOLENKO Рекомендовано Вченою радою Української медичної стоматологічної академії як навчальний посібник для іноземних студентів – здобувачів вищої освіти ступеня магістра, які навчаються за спеціальністю 221 «Стоматологія» у закладах вищої освіти МОЗ України (протокол №8 від 11.03.2020р) Reviewers Romanuk A. - MD, Professor, Head of the Department of Pathological Anatomy, Sumy State University. Sitnikova V. - MD, Professor of Department of Normal and Pathological Clinical Anatomy Odessa National Medical University. Yeroshenko G. - MD, Professor, Department of Histology, Cytology and Embryology Ukrainian Medical Dental Academy. A teaching manual in English, developed at the Department of Pathological Anatomy with a section course UMSA by Professor Starchenko II, Associative Professor Prylutsky OK, Assistant Zadvornova AP, Assistant Nikolenko DE. The manual presents the content and basic questions of the topic, practical skills in sufficient volume for each class to be mastered by students, algorithms for describing macro- and micropreparations, situational tasks. The formulation of tests, their number and variable level of difficulty, sufficient volume for each topic allows to recommend them as preparation for students to take the licensed integrated exam "STEP-1". 2 Contents p. 1 Introduction to pathomorphology. Subject matter and tasks of 5 pathomorphology. Main stages of development of pathomorphology. Methods of pathanatomical diagnostics. Methods of pathomorphological research. 2 Morphological changes of cells as response to stressor and toxic damage 8 (parenchimatouse / intracellular dystrophies). -

Paraneoplastic Syndrome Presenting As Giant Porokeratosis in a Patient with Nasopharyngeal Cancer
Paraneoplastic Syndrome Presenting As Giant Porokeratosis in A Patient with Nasopharyngeal Cancer Fitri Azizah, Sonia Hanifati, Sri Adi Sularsito, Lili Legiawati, Shannaz Nadia Yusharyahya, Rahadi Rihatmadja Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo National General Hospital Keywords: porokeratosis, giant porokeratosis, paraneoplastic syndrome, nasopharyngeal Abstract: Giant porokeratosis is a rare condition in which the hyperkeratotic plaques of porokeratosis reach up to 20 cm in diameter. Porokeratosis is characterized clinically by hyperkeratotic papules or plaques with a thread-like elevated border. Although rare, porokeratosis has been reported in conjunction with malignancies suggesting a paraneoplastic nature. Associated malignancies reported were hematopoietic, hepatocellular, and cholangiocarcinoma. We report a case of giant porokeratosis in a patient with nasopharyngeal cancer responding to removal of the primary cancer by chemoradiotherapy. 1 INTRODUCTION regress completely after the treatment of malignancy, suggestive of paraneoplastic syndrome. Porokeratosis is a chronic progressive disorder of keratinization, characterized by hyperkeratotic papules or plaques surrounded by a thread-like 2 CASE elevated border corresponds to a typical histologic hallmark, the cornoid lamella . O regan, 2012) There Mr. SS, 68-year-old, was referred for evaluation of are at least six clinical variants of porokeratosis pruritic, slightly erythematous plaques with raised, recognized with known genetic disorder.1 Some hyperpigmented border of one and a half year clinical variant of porokeratosis has been reported in duration on the extensor surface of both legs. The the setting of immunosuppressive conditions, organ lesions shown minimal response to potent topical transplantation, use of systemic corticosteroids, and corticosteroids and phototherapy given during the infections, suggesting that impaired immunity may last 8 months in another hospital. -

ORIGINAL ARTICLE a Clinical and Histopathological Study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka
ORIGINAL ARTICLE A Clinical and Histopathological study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka. Khaled A1, Banu SG 2, Kamal M 3, Manzoor J 4, Nasir TA 5 Introduction studies from other countries. Skin diseases manifested by lichenoid eruption, With this background, this present study was is common in our country. Patients usually undertaken to know the clinical and attend the skin disease clinic in advanced stage histopathological pattern of lichenoid eruption, of disease because of improper treatment due to age and sex distribution of the diseases and to difficulties in differentiation of myriads of well assess the clinical diagnostic accuracy by established diseases which present as lichenoid histopathology. eruption. When we call a clinical eruption lichenoid, we Materials and Method usually mean it resembles lichen planus1, the A total of 134 cases were included in this study prototype of this group of disease. The term and these cases were collected from lichenoid used clinically to describe a flat Bangabandhu Sheikh Mujib Medical University topped, shiny papular eruption resembling 2 (Jan 2003 to Feb 2005) and Apollo Hospitals lichen planus. Histopathologically these Dhaka (Oct 2006 to May 2008), both of these are diseases show lichenoid tissue reaction. The large tertiary care hospitals in Dhaka. Biopsy lichenoid tissue reaction is characterized by specimen from patients of all age group having epidermal basal cell damage that is intimately lichenoid eruption was included in this study. associated with massive infiltration of T cells in 3 Detailed clinical history including age, sex, upper dermis. distribution of lesions, presence of itching, The spectrum of clinical diseases related to exacerbating factors, drug history, family history lichenoid tissue reaction is wider and usually and any systemic manifestation were noted. -

Immunologic Adverse Reactions of Β-Blockers and the Skin (Review)
EXPERIMENTAL AND THERAPEUTIC MEDICINE 18: 955-959, 2019 Immunologic adverse reactions of β-blockers and the skin (Review) ALIN LAURENTIU TATU1, ALINA MIHAELA ELISEI1, VALENTIN CHIONCEL2, MAGDALENA MIULESCU3 and LAWRENCE CHUKWUDI NWABUDIKE4 1Medical and Pharmaceutical Research Unit/Competitive, Interdisciplinary Research Integrated Platform ‘Dunărea de Jos’, ReForm-UDJG; Research Centre in the Field of Medical and Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, Department of Pharmaceutical Sciences, ‘Dunărea de Jos’ University of Galați, 800010 Galati; 2Department of Cardio-Thoracic Pathology, Faculty of Medicine, ‘Carol Davila’ University of Medicine and Phamacy, 050474 Bucharest; 3Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, ‘Dunarea de Jos University’ of Galati, 800010 Galati; 4Department of Diabetic Foot Care, ‘Prof. N. Paulescu’ National Institute of Diabetes, 011233 Bucharest, Romania Received September 11, 2018; Accepted November 16, 2018 DOI: 10.3892/etm.2019.7504 Abstract. β-Blockers are a widely utilised class of medica- use, as well as possible therapeutic approaches to these. This tion. They have been in use for a variety of systemic disorders short review will focus on those dermatoses resulting from including hypertension, heart failure and intention tremors. β-blocker use, which have an immunologic basis. Their use in dermatology has garnered growing interest with the discovery of their therapeutic effects in the treatment of haemangiomas, their potential positive effects in wound Contents healing, Kaposi sarcoma, melanoma and pyogenic granuloma, and, more recently, pemphigus. Since β-blockers are deployed 1. Introduction in a variety of disorders, which have cutaneous co-morbidities 2. Cutaneous side - effects of β-blockers such as psoriasis, their pertinence to dermatologists cannot be 3. -

Atypical Variants of Mycosis Fungoides
Altınışık et al. Atypical Variants of Mycosis Fungoides REVIEW DOI: 10.4274/jtad.galenos.2021.09719 J Turk Acad Dermatol 2021;15(2):30-33 Atypical Variants of Mycosis Fungoides Dursun Dorukhan Altınışık, Özge Aşkın, Tuğba Kevser Uzunçakmak, Burhan Engin Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Dermatology, Istanbul, Turkey ABSTRACT Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and it is characterised by specific histological features. MF is in a spectrum of disorders which includes Sezary syndrome and other CTCL subtypes. Clinically and histopathologically it can imitate other T-cell mediated dermatosis hence making its diagnosis difficult. Due to different prognosis and difference in treatment strategies other subtypes of CTCL must be differentiated from MF. MF is a clinically indolent low-grade lymphoma which is seen in people aged between 55- 60 and it’s seen more commonly in men (2:1 ratio). Meanwhile other subtypes of MF like folliculotropic variant may show relatively a more aggressive course and our treatment strategy may differ. Keywords: Mycosis fungoides, Cutaneous T-cell lymphoma, Phototherapy, PUVA Introduction follicles frequently causing alopecia and in the paediatric population Mycosis fungoides (MF) is the most common type of cutaneous T-cell it tends to accompany hypopigmented patches and plaques. FMF lymphoma (CTCL) and it is characterised by specific histological preferentially involves the head and neck region however it can features [1]. MF is in a spectrum of disorders which includes Sezary sometimes involve the trunk or the extremities [6]. Patients with syndrome and other CTCL subtypes. Clinically and histopathologically FMF have significant pruritus which may be overlapped by S. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Lichen Spinulosus: Case Report and Review of Literatures
Journal of Health Science 2015, 5(3A): 20-22 DOI: 10.5923/s.health.201501.07 Lichen Spinulosus: Case Report and Review of Literatures Khalid Al Hawsawi1,*, Kholood Almehmadi2, Bushra Alraddadi3, Ohood Aljuhani2 1Dermatology Consultant, Head of Dermatology Department, King Abdul Aziz Hospital, Makkah, Saudi Arabia 2Medical intern, King Abdul Aziz Hospital, Makkah, Saudi Arabia 3Dermatology Resident, King Abdul Aziz Hospital, Makkah, Saudi Arabia Abstract Lichen Spinulosus is a rare childhood disease. It is characterized by follicular hyperkeratotic papules that may coalesce into plaques. Individual lesion shows conical projection, hair like horny spine. The etiology is unknown but genetic predisposition has been suggested as a possible cause. It has a tendency to disappear spontaneously at puberty. Herein we present a 12-year-old boy presented with 6- month- history of persistent asymptomatic skin lesions. Skin examinations revealed multiple grouped scaly, hypopigmented tiny follicular papules with horny spines on his back. Soles showed planter keratoderma. Skin biopsy showed dilated hair follicle filled with keratin plugging. Diagnosis of LS associated with planter keratoderma was made. The patient responded well to topical lactic acid (12%), urea (20-30%), and betamethasone valerate (0.1%) preparations. Keywords Lichen spinulosa, Lichen Spinulosus 1. Introduction appearance. LS usually involves extensor surfaces of arms, trochanteric regions, neck, abdomen, buttocks, thigh, Lichen spinulosus (LS) is a genetic disorder of popliteal fossae, and knees. The histopathology of the lesion is not specific showing dilated hair follicle with keratin keratinization of hair follicles. LS was first described by Adamson in 1908. [1] It is a member of the family of plugging. -

Differentiating Keratosis Follicularis Spinulosa Decalvans from Monilithrix : Two Rare Causes of Scarring Alopecia in Young Male Siblings
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 7 (May. 2019), PP 01-03 www.iosrjournals.org Differentiating Keratosis Follicularis Spinulosa Decalvans From Monilithrix : Two Rare Causes Of Scarring Alopecia In Young Male Siblings. 1 2 3 4 5 Heena Singdia , Rohit Garg , Sinni Jain , Vijay Paliwal , Puneet Bhargava , Deepak Mathur6 1,2,3,4,5,6(Department of dermatology, venereology and leprology, SMS Medical College, India) Corresponding Author: Heena Singdia Abstract: Keratosis follicularis spinulosa decalvans (KFSD) is a rare disorder affecting the hair follicles characterized by progressive cicatricial alopecia of the scalp and eyebrows, with usually X-linked recessive mode of transmission hence predominantly affecting Males.[1] Monilethrix is a rare structural Hair shaft disorder with autosomal dominant mode of transmission, characterized by short, fragile, brittle hair that breaks spontaneously resulting in patchy dystrophic alopecia.[2] Key words: Keratosis Follicularis Spinulosa Decalvans, Monilithrix, Hereditary Scarring Alopecias --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 02-05-2019 Date of acceptance: 16-05-2019 --------------------------------------------------------------------------------------------------------------------------------------------------- I. Introduction KFSD is a rare type of primary scarring alopecia with lymphocytic predominance. -

My Approach to Superficial Inflammatory Dermatoses K O Alsaad, D Ghazarian
1233 J Clin Pathol: first published as 10.1136/jcp.2005.027151 on 25 November 2005. Downloaded from REVIEW My approach to superficial inflammatory dermatoses K O Alsaad, D Ghazarian ............................................................................................................................... J Clin Pathol 2005;58:1233–1241. doi: 10.1136/jcp.2005.027151 Superficial inflammatory dermatoses are very common and diagnosis of inflammatory skin diseases, there are limitations to this approach. The size of the comprise a wide, complex variety of clinical conditions. skin biopsy should be adequate and representa- Accurate histological diagnosis, although it can sometimes tive of all four compartments and should also be difficult to establish, is essential for clinical include hair follicles. A 2 mm punch biopsy is too small to represent all compartments, and often management. Knowledge of the microanatomy of the skin insufficient to demonstrate a recognisable pat- is important to recognise the variable histological patterns tern. A 4 mm punch biopsy is preferred, and of inflammatory skin diseases. This article reviews the non- usually adequate for the histological evaluation of most inflammatory dermatoses. However, a vesiculobullous/pustular inflammatory superficial larger biopsy (6 mm punch biopsy), or even an dermatoses based on the compartmental microanatomy of incisional biopsy, might be necessary in panni- the skin. culitis or cutaneous lymphoproliferative disor- ders. A superficial or shave biopsy should be .......................................................................... -
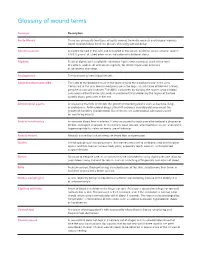
Wounds Definitions
Glossary of wound terms Acronym Description Acute Wound There are principally two types of acute wound; traumatic wounds and surgical wounds. Acute wounds follow the three phases of healing without delay. Albumin (serum) A protein formed in the liver and circulated in the serum. A normal serum albumin level is 3.5-5.0 grams/ dl. Used often as an indication of nutritional status. Alginate A salt of alginic acid, a colloidal substance from brown seaweed; used, in the form of calcium, sodium, or ammonium alginate, for dental impression materials or absorptive dressings. Angiogenesis The formation of new blood vessels. Ankle-Brachial Index (ABI) The ratio of the blood pressure in the lower legs to the blood pressure in the arms. Compared to the arm, lower blood pressure in the leg is an indication of blocked arteries (peripheral vascular disease). The ABI is calculated by dividing the higher systolic blood pressure in either the dorsalis pedis or posterior tibial arteries by the higher of the two systolic blood pressures in the arm. Antimicrobial agents A substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes (microbicidal) or prevent the growth of microbes (microbistatic). Disinfectants are antimicrobial substances used on non-living objects. Arterial insufficiency Inadequate blood flow in arteries. It may be caused by occlusive atherosclerotic plaques or emboli; damaged, diseased, or intrinsically weak vessels; arteriovenous fistulas; aneurysms; hypercoagulability states; or heavy use of tobacco. Arterial wound Wounds caused by lack of adequate blood flow and perfusion. Biofilm A thick grouping of microorganisms that are very resistant to antibiotics and antimicrobial agents and that lives on various body parts, especially teeth, wounds, and implanted surgical devices. -

Imatinib-Induced Lichenoid Eruption: a Case Report and a Review of Literature
Vol.33 No.4 Case report 329 Imatinib-induced lichenoid eruption: A case report and a review of literature. Thiraphong Mekwilaiphan MD, Natta Rajatanavin MD. ABSTRACT: MEKWILAIPHAN T, RAJATANAVIN N. IMATINIB-INDUCED LICHENOID ERUPTION: A CASE REPORT AND A REVIEW OF LITERATURE. THAI J DERMATOL 2017;33: 329-335. DIVISION OF DERMATOLOGY, DEPARTMENT OF MEDICINE, FACULTY OF MEDICINE, RAMATHIBODI HOSPITAL, MAHIDOL UNIVERSITY, BANGKOK, THAILAND. Imatinib is the first generation tyrosine kinase inhibitor which consisted of bcr-abl, c-kit, and platelet-derived growth factor receptors (PDGFRs). Its tolerability in the treatment of malignancies is higher than the conventional chemotherapy. However, various adverse cutaneous reactions are the most common side effect of imatinib. Nevertheless, lichenoid eruption is an uncommon cutaneous reaction from imatinib. The clinical manifestation is violaceous, flat-topped papules or plaques involving mainly trunk and extremities. Oral and genital mucosal involvement is a distinctive feature. There were no specific histopathological findings, but usually not a typical characteristic of lichen planus. Due to high prevalence of cutaneous rash, the pathogenesis may be related to pharmacological effect than hypersensitivity reaction. Dosage decrement of imatinib is a choice of treatment, or switch to the second or third generation tyrosine kinase inhibitors. Acitretin was successfully used to treat imatinib-induced lichenoid eruption in our patient and enabling the continuation of the high imatinib dosage for her hematologic malignancy. Key words: Imatinib, lichenoid eruption, tyrosine kinase inhibitor From: Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University Corresponding author: Thiraphong Mekwilaiphan MD., email: [email protected] 330 Mekwilaiphan T et al Thai J Dermatol, October-December, 2017 บทคัดยอ: ธีรพงษ เมฆวิไลพันธุ, ณัฏฐา รัชตะนาวิน รายงานการเกิดผื่นชนิดไลเคนนอยด ในผูปวยที่ไดรับยาอิมมาทินิบ และ การทบทวนวรรณกรรม วารสารโรคผิวหนัง 2560; 33: 329-335. -

Progressive Widespread Warty Skin Growths
DERMATOPATHOLOGY DIAGNOSIS Progressive Widespread Warty Skin Growths Patrick M. Kupiec, BS; Eric W. Hossler, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 33-year-old man presented with progres- sive widespread warty skin growths that had been present copysince 6 years of age. Physical examination revealed numerous verrucous papules on the face and neck along with Figure 1. H&E, original magnification ×40. Figure 2. H&E, original magnification ×40. verrucous, tan-pink papules and plaques diffuselynot scattered on the trunk, arms, and legs. A biopsy of a lesion on the neck Dowas performed. H&E, original magnification ×200. The best diagnosisCUTIS is: a. condyloma acuminatum b. epidermodysplasia verruciformis c. herpesvirus infection d. molluscum contagiosum e. verruca vulgaris PLEASE TURN TO PAGE 99 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Mr. Kupiec is from the State University of New York (SUNY) Upstate Medical University, Syracuse. Dr. Hossler is from the Departments of Dermatology and Pathology, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Patrick M. Kupiec, BS, 50 Presidential Plaza, Syracuse, NY 13202 ([email protected]). 82 CUTIS® WWW.CUTIS.COM Copyright Cutis 2017. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher.