Spatial Relation of Stem Hydroids to Branch Hydroids in Four Pleurocarpous Mosses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alberta Wetland Classification System – June 1, 2015
Alberta Wetland Classification System June 1, 2015 ISBN 978-1-4601-2257-0 (Print) ISBN: 978-1-4601-2258-7 (PDF) Title: Alberta Wetland Classification System Guide Number: ESRD, Water Conservation, 2015, No. 3 Program Name: Water Policy Branch Effective Date: June 1, 2015 This document was updated on: April 13, 2015 Citation: Alberta Environment and Sustainable Resource Development (ESRD). 2015. Alberta Wetland Classification System. Water Policy Branch, Policy and Planning Division, Edmonton, AB. Any comments, questions, or suggestions regarding the content of this document may be directed to: Water Policy Branch Alberta Environment and Sustainable Resource Development 7th Floor, Oxbridge Place 9820 – 106th Street Edmonton, Alberta T5K 2J6 Phone: 780-644-4959 Email: [email protected] Additional copies of this document may be obtained by contacting: Alberta Environment and Sustainable Resource Development Information Centre Main Floor, Great West Life Building 9920 108 Street Edmonton Alberta Canada T5K 2M4 Call Toll Free Alberta: 310-ESRD (3773) Toll Free: 1-877-944-0313 Fax: 780-427-4407 Email: [email protected] Website: http://esrd.alberta.ca Alberta Wetland Classification System Contributors: Matthew Wilson Environment and Sustainable Resource Development Thorsten Hebben Environment and Sustainable Resource Development Danielle Cobbaert Alberta Energy Regulator Linda Halsey Stantec Linda Kershaw Arctic and Alpine Environmental Consulting Nick Decarlo Stantec Environment and Sustainable Resource Development would also -

Clonal Structure in the Moss, Climacium Americanum Brid
Heredity 64 (1990) 233—238 The Genetical Society of Great Britain Received 25August1989 Clonal structure in the moss, Climacium americanum Brid. Thomas R. Meagher* and * Departmentof Biological Sciences, Rutgers University, P.O. Box 1059, Jonathan Shawt Piscataway, NJ 08855-1059. 1 Department of Biology, Ithaca College, Ithaca, NY 14850. The present study focussed on the moss species, Climacium americanum Brid., which typically grows in the form of mats or clumps of compactly spaced gametophores. In order to determine the clonal structure of these clumps, the distribution of genetic variation was analysed by an electrophoretic survey. Ten individual gametophores from each of ten clumps were sampled in two locations in the Piedmont of North Carolina. These samples were assayed for allelic variation at six electrophoretically distinguishable loci. For each clump, an explicit probability of a monomorphic versus a polymorphic sample was estimated based on allele frequencies in our overall sample. Although allelic variation was detected within clumps at both localities, our statistical results showed that the level of polymorphism within clumps was less than would be expected if genetic variation were distributed at random within the overall population. INTRODUCTION approximately 50 per cent of moss species that are hermaphroditic, self-fertilization of bisexual Ithas been recognized that in many taxa asexual gametophytes results in completely homozygous propagation as the predominant means of repro- sporophytes. Dispersal of such genetically -

Household and Personal Uses
Glime, J. M. 2017. Household and Personal Uses. Chapt. 1-1. In: Glime, J. M. Bryophyte Ecology. Volume 5. Uses. Ebook sponsored 1-1-1 by Michigan Technological University and the International Association of Bryologists. Last updated 5 October 2017 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1 HOUSEHOLD AND PERSONAL USES TABLE OF CONTENTS Household Uses...................................................................................................................................................1-1-2 Furnishings...................................................................................................................................................1-1-4 Padding and Absorption...............................................................................................................................1-1-5 Mattresses.............................................................................................................................................1-1-6 Shower Mat...........................................................................................................................................1-1-7 Urinal Absorption.................................................................................................................................1-1-8 Cleaning.......................................................................................................................................................1-1-8 Brushes and Brooms.............................................................................................................................1-1-8 -

Climacium Dendroides from Cofre De Perote, a High-Elevation Tropical Montane Site in Veracruz, Mexico
The Bryologist 107(3), pp. 368 372 Copyright q 2004 by the American Bryological and Lichenological Society, Inc. Climacium dendroides from Cofre de Perote, A High-Elevation Tropical Montane Site in Veracruz, Mexico ANGELA E. NEWTON Department of Botany, Natural History Museum, London SW7 5BD, United Kingdom; e-mail: a.newton@nhm. ac.uk EFRAIÂN DE LUNA Instituto de EcologõÂa, A.C., Ap. Postal 63, 91000 Xalapa, Veracruz, MeÂxico; e-mail: [email protected] Abstract. Climacium dendroides (Hedw.) F. Weber & D. Mohr is reported new to Mexico and in the ®rst known locality in the tropics, at the elevation of 3,000 m, on the ancient shield volcano of Cofre de Perote. Variation in branching of the costa is assessed in this plant and in material of C. dendroides worldwide. Additional taxa of interest from this locality on Cofre de Perote include Aulacomnium palustre and Polytrichum commune (both new to the state of Veracruz). Keywords. `Boreal' mosses, Climacium, distribution, Mexico, montane tropics. The ancient shield volcano of Cofre de Perote is 20 m across, and consisted of a series of small located in the Neovolcanic Axis of Mexico, at swamps connected by the small stream. Climacium 198329 N, 978309 W, and reaches an elevation of was growing on low hummocks to one side of the 4,200 m. The upper regions of the mountain have stream, and the plants were deeply embedded in the extensive Pinus-Abies forests with a rich ¯ora of surrounding mosses and other plants. Additional mosses, ferns, and cycads, that has been the focus moss species in the immediate area included Po- of much botanical study in the past, including the lytrichum commune Hedw. -

Field Guide to the Moss Genera in New Jersey by Keith Bowman
Field Guide to the Moss Genera in New Jersey With Coefficient of Conservation and Indicator Status Keith Bowman, PhD 10/20/2017 Acknowledgements There are many individuals that have been essential to this project. Dr. Eric Karlin compiled the initial annotated list of New Jersey moss taxa. Second, I would like to recognize the contributions of the many northeastern bryologists that aided in the development of the initial coefficient of conservation values included in this guide including Dr. Richard Andrus, Dr. Barbara Andreas, Dr. Terry O’Brien, Dr. Scott Schuette, and Dr. Sean Robinson. I would also like to acknowledge the valuable photographic contributions from Kathleen S. Walz, Dr. Robert Klips, and Dr. Michael Lüth. Funding for this project was provided by the United States Environmental Protection Agency, Region 2, State Wetlands Protection Development Grant, Section 104(B)(3); CFDA No. 66.461, CD97225809. Recommended Citation: Bowman, Keith. 2017. Field Guide to the Moss Genera in New Jersey With Coefficient of Conservation and Indicator Status. New Jersey Department of Environmental Protection, New Jersey Forest Service, Office of Natural Lands Management, Trenton, NJ, 08625. Submitted to United States Environmental Protection Agency, Region 2, State Wetlands Protection Development Grant, Section 104(B)(3); CFDA No. 66.461, CD97225809. i Table of Contents Introduction .................................................................................................................................................. 1 Descriptions -

Water Relations: Conducting Structures
Glime, J. M. 2017. Water Relations: Conducting Structures. Chapt. 7-1. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological 7-1-1 Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 7 March 2017 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 7-1 WATER RELATIONS: CONDUCTING STRUCTURES TABLE OF CONTENTS Movement to Land .............................................................................................................................................. 7-1-2 Bryophytes as Sponges ....................................................................................................................................... 7-1-2 Conducting Structure .......................................................................................................................................... 7-1-4 Leptomes and Hydromes............................................................................................................................. 7-1-8 Hydroids............................................................................................................................................. 7-1-12 Leptoids.............................................................................................................................................. 7-1-14 Rhizome..................................................................................................................................................... 7-1-15 Leaves....................................................................................................................................................... -

A Baseline Study of Alpine Snowbed and Rill Communities on Mount Washington, Nh Author(S): Robert S
A Baseline Study of Alpine Snowbed and Rill Communities On Mount Washington, Nh Author(s): Robert S. Capers Nancy G. Slack Source: Rhodora, 118(976):345-381. Published By: The New England Botanical Club, Inc. DOI: http://dx.doi.org/10.3119/16-07 URL: http://www.bioone.org/doi/full/10.3119/16-07 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. RHODORA, Vol. 118, No. 976, pp. 345–381, 2016 Ó Copyright 2016 by the New England Botanical Club doi: 10.3119/16-07; first published on-line February 6, 2017. A BASELINE STUDY OF ALPINE SNOWBED AND RILL COMMUNITIES ON MOUNT WASHINGTON, NH ROBERT S. CAPERS Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269 e-mail: [email protected] NANCY G. SLACK Biology Department, The Sage Colleges, 65 1st St., Troy, NY 12180 ABSTRACT. -

Observations on Stem Surface Anatomy In
Arctoa (2000) 9: 151-154 OBSERVATIONS ON STEM SURFACE ANATOMY IN CLIMACIUM AND PLEUROZIOPSIS (CLIMACIACEAE: MUSCI) Î ÑÒÐÎÅÍÈÈ ÏÎÂÅÐÕÍÎÑÒÈ ÑÒÅÁËß CLIMACIUM È PLEUROZIOPSIS (CLIMACIACEAE: MUSCI) DANIEL H. NORRIS1 & MICHAEL S. IGNATOV2 ÄÀÍÈÝËÜ Ã. ÍÎÐÐÈÑ1 & ÌÈÕÀÈË Ñ. ÈÃÍÀÒÎÂ2 Abstract Pleuroziopsis was segregated from Climaciaceae into a separate monotypic family Pleuroziopsidaceae by Ireland (1968). According to Ireland, the most important and unique characteristic of Pleuroziopsis is a fluting of the stem by streaks of stem lamellae. However, this pattern of stem fluting is shown to be shared with the genus Climacium, so Pleuroziopsis is returned to Climaciaceae. Ðåçþìå Pleuroziopsis áûë âûäåëåí èç ñåìåéñòâà Climaciaceae â ñàìîñòîÿòåëüíîå ìîíîòèïíîå ñåìåéñòâî Pleuroziopsidaceae (Ireland, 1968). Îñíîâàíèåì äëÿ ýòîãî ïîñëóæèëî íàëè÷èå ïðîäîëüíûõ ‘ñòåáëåâûõ ïëàñòèí’, ñòðóêòóðû, êàê ñ÷èòàë Àéðåëàíä, íå èìåþùåé ãîìîëîãîâ. Îäíàêî, äàííîå èññëåäîâàíèå âûÿâèëî, ÷òî ñòðóêòóðà ïîâåðõíîñòè ñòåáëÿ ó Climacium è Pleuroziopsis ïðèíöèïèàëüíî ñõîäíà, òàê ÷òî ïîñëåäíèé ðîä ïðåäëàãàåòñÿ âåðíóòü â Climaciaceae. Many years ago, I (DHN) found myself with anatomy was first thought to allow easy sorting R. Ochyra, Krakow, in several very profitable dis- according to the observations of Ireland (1968). I cussions on paraphyllial arrangement in Hyp- (DHN) removed the leaves from both the stem nobryalean mosses. These discussions led in sub- and rhizome material of the two species. At that sequent years to careful observation of the pat- point, the cloaking of rhizoids was so dense as to terns of paraphyllial insertion of moss stems. A require plucking of those rhizoids with fine-point recent trip by DHN to Alaska afforded oppor- forceps. It was with great surprise that we found tunity to study freshly collected material from the denuded stems (split longitudinally to allow a population of Climacium dendroides (Hedw.) increased transparency) to be essentially identi- Web. -
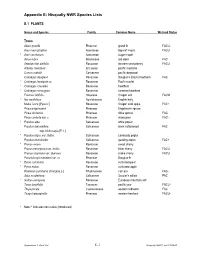
Appendix E Nisqually Species List
Appendix E: Nisqually NWR Species Lists E.1 PLANTS Genus and Species Family Common Name Wetland Status Trees Abies grandis Pinaceae grand fir FACU- Acer macrophyllum Aceraceae big-leaf maple FACU * Acer saccharum Aceraceae sugar maple Alnus rubra Betulaceae red alder FAC Amelanchier alnifolia Rosaceae western serviceberry FACU Arbutus menziesii Ericaceae pacific madrone Cornus nuttallii Cornaceae pacific dogwood Crataegus douglasii Rosaceae Douglas’s (black) hawthorn FAC * Crataegus laevigata cv. Rosaceae Paul's scarlet * Crataegus x lavallei Rosaceae hawthorn * Crataegus monogyna Rosaceae common hawthorn Fraxinus latifolia Oleaceae Oregon ash FACW * Ilex aquifolium Aquifoliaceae English holly Malus fusca [Pyrus f.] Rosaceae Oregon crab apple FAC+ Picea engelmannii Pinaceae Engelmann spruce Picea sitchensis Pinaceae Sitka spruce FAC Pinus contorta var. c. Pinaceae shore pine FAC- * Populus alba Salicaceae white poplar Populus balsamifera Salicaceae black cottonwood FAC ssp. trichocarpa [P. t. ] * Populus nigra var. italica Salicaceae Lombardy poplar Populus tremuloides Salicaceae quaking aspen FAC+ * Prunus avium Rosaceae sweet cherry Prunus emarginata var. mollis Rosaceae bitter cherry FACU Prunus virginiana var. demissa Rosaceae choke cherry FACU Pseudotsuga menziesii var. m. Pinaceae Douglas-fir * Pyrus communis Rosaceae cultivated pear * Pyrus malus Rosaceae cultivated apple Rhamnus purshiana [Frangula p.] Rhamnaceae cascara FAC- Salix scouleriana Salicaceae Scouler’s willow FAC * Sorbus aucuparia Rosaceae European mountain ash Taxus brevifolia Taxaceae pacific yew FACU- Thuja plicata Cupressaceae western redcedar FAC Tsuga heterophylla Pinaceae western hemlock FACU- * Note: * indicates non-native (introduced) Appendix E.1: Plant List E-1 Nisqually NWR Final CCP/EIS Genus and Species Family Common Name Wetland Status Shrubs, Brambles & Vines Acer circinatum Aceraceae vine maple FACU+ Arctostaphylos uva-ursi var. -

Spoon-Leaved Moss Bryoandersonia Illecebra
COSEWIC Assessment and Status Report on the Spoon-leaved Moss Bryoandersonia illecebra in Canada ENDANGERED 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION DES ENDANGERED WILDLIFE ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and status report on the spoon-leaved moss Bryoandersonia illecebra in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 31 pp. Doubt, J 2003. COSEWIC status report on the spoon-leaved moss Bryoandersonia illecebra in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-31 pp. Production note: COSEWIC would like to acknowledge Jennifer C. Doubt for writing the status report on the spoon-leaved moss Bryoandersonia illecebra, prepared under the contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur la situation de l’andersonie charmante (Bryoandersonia illecebra) au Canada Cover illustration: Spoon-leaved moss — Provided by the author. Her Majesty the Queen in Right of Canada 2003 Catalogue No. CW69-14/331-2003E-PDF ISBN 0-662-35350-1 HTML: CW69-14/331-2003E-HTML 0-662-35351-X Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2003 Common name Spoon-leaved moss Scientific name Bryoandersonia illecebra Status Endangered Reason for designation This species is endemic to eastern North America. -

Biodiversity
Appendix I Biodiversity Appendix I1 Literature Review – Biodiversity Resources in the Oil Sands Region of Alberta Syncrude Canada Ltd. Mildred Lake Extension Project Volume 3 – EIA Appendices December 2014 APPENDIX I1: LITERATURE REVIEW – BIODIVERSITY RESOURCES IN THE OIL SANDS REGION OF ALBERTA TABLE OF CONTENTS PAGE 1.0 BIOTIC DIVERSTY DATA AND SUMMARIES ................................................................ 1 1.1 Definition ............................................................................................................... 1 1.2 Biodiversity Policy and Assessments .................................................................... 1 1.3 Environmental Setting ........................................................................................... 2 1.3.1 Ecosystems ........................................................................................... 2 1.3.2 Biota ...................................................................................................... 7 1.4 Key Issues ............................................................................................................. 9 1.4.1 Alteration of Landscapes and Landforms ............................................. 9 1.4.2 Ecosystem (Habitat) Alteration ........................................................... 10 1.4.3 Habitat Fragmentation and Edge Effects ............................................ 10 1.4.4 Cumulative Effects .............................................................................. 12 1.4.5 Climate Change ................................................................................. -

Allenberg Bog Plant List
ALLENBERG BOG AUDUBON NATURE PRESERVE Allenberg Bog is also known to some as Waterman's Swamp, Congdon's Pond, and Owlenburg Bog and is on the border of the towns of Napoli and New Albion, New York in Cattaraugus County. A unique and fascinating refuge of 390 acres, it is the jewel of the Buffalo Audubon Preserve System. Even before the first parcels joined Audubon's preserve holdings in 1957, the area was famous among botanists for its wild orchids, more than 30 species of liverworts, nearly 60 species of mosses, and approximately 258 species of vascular plants. It should be noted that any collecting of any plants in this or any of our refuges is strictly prohibited. Please respect the purposes behind "Preserves." Plant List: Liverworts Family Ptilidiaceae Trichocolea tomentella Family Lepidoziaceae Bazzania trilobata Family Calypogeiaceae Calypogeia neesiana Calypogeia sphagnicola Calypogeia trichomanis Family Cephaloziaceae Cephalozia connivens Cephalozia media Cladopodiella fluitans Family Jungermanniaceae Lophoxia gracilis Jamesoniella autumnalis Plectocolea crenulata Family Harpanthaceae Lophocolea heterophylla Chiloscyphus pallescens Harpanthus scutatus Geocalyx graveolans Family Porellaceae Porella platyphylloides Family Radulaceae Radula complanata Family Frullaniaceae Frullania asagrayana Frullania brittoniae Frullania eboracensis Frullania oakesiana Frullania tamirisci Family Pelliaceae Pellia jabbroniana Family Pallavicniaceae Pallavicinia lyelli Family Riccardiaceae Riccardia latrifons Riccardia multifida Family Marchantiaceae