Clonal Structure in the Moss, Climacium Americanum Brid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Household and Personal Uses
Glime, J. M. 2017. Household and Personal Uses. Chapt. 1-1. In: Glime, J. M. Bryophyte Ecology. Volume 5. Uses. Ebook sponsored 1-1-1 by Michigan Technological University and the International Association of Bryologists. Last updated 5 October 2017 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1 HOUSEHOLD AND PERSONAL USES TABLE OF CONTENTS Household Uses...................................................................................................................................................1-1-2 Furnishings...................................................................................................................................................1-1-4 Padding and Absorption...............................................................................................................................1-1-5 Mattresses.............................................................................................................................................1-1-6 Shower Mat...........................................................................................................................................1-1-7 Urinal Absorption.................................................................................................................................1-1-8 Cleaning.......................................................................................................................................................1-1-8 Brushes and Brooms.............................................................................................................................1-1-8 -

Field Guide to the Moss Genera in New Jersey by Keith Bowman
Field Guide to the Moss Genera in New Jersey With Coefficient of Conservation and Indicator Status Keith Bowman, PhD 10/20/2017 Acknowledgements There are many individuals that have been essential to this project. Dr. Eric Karlin compiled the initial annotated list of New Jersey moss taxa. Second, I would like to recognize the contributions of the many northeastern bryologists that aided in the development of the initial coefficient of conservation values included in this guide including Dr. Richard Andrus, Dr. Barbara Andreas, Dr. Terry O’Brien, Dr. Scott Schuette, and Dr. Sean Robinson. I would also like to acknowledge the valuable photographic contributions from Kathleen S. Walz, Dr. Robert Klips, and Dr. Michael Lüth. Funding for this project was provided by the United States Environmental Protection Agency, Region 2, State Wetlands Protection Development Grant, Section 104(B)(3); CFDA No. 66.461, CD97225809. Recommended Citation: Bowman, Keith. 2017. Field Guide to the Moss Genera in New Jersey With Coefficient of Conservation and Indicator Status. New Jersey Department of Environmental Protection, New Jersey Forest Service, Office of Natural Lands Management, Trenton, NJ, 08625. Submitted to United States Environmental Protection Agency, Region 2, State Wetlands Protection Development Grant, Section 104(B)(3); CFDA No. 66.461, CD97225809. i Table of Contents Introduction .................................................................................................................................................. 1 Descriptions -

Water Relations: Conducting Structures
Glime, J. M. 2017. Water Relations: Conducting Structures. Chapt. 7-1. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological 7-1-1 Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 7 March 2017 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 7-1 WATER RELATIONS: CONDUCTING STRUCTURES TABLE OF CONTENTS Movement to Land .............................................................................................................................................. 7-1-2 Bryophytes as Sponges ....................................................................................................................................... 7-1-2 Conducting Structure .......................................................................................................................................... 7-1-4 Leptomes and Hydromes............................................................................................................................. 7-1-8 Hydroids............................................................................................................................................. 7-1-12 Leptoids.............................................................................................................................................. 7-1-14 Rhizome..................................................................................................................................................... 7-1-15 Leaves....................................................................................................................................................... -

A Baseline Study of Alpine Snowbed and Rill Communities on Mount Washington, Nh Author(S): Robert S
A Baseline Study of Alpine Snowbed and Rill Communities On Mount Washington, Nh Author(s): Robert S. Capers Nancy G. Slack Source: Rhodora, 118(976):345-381. Published By: The New England Botanical Club, Inc. DOI: http://dx.doi.org/10.3119/16-07 URL: http://www.bioone.org/doi/full/10.3119/16-07 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. RHODORA, Vol. 118, No. 976, pp. 345–381, 2016 Ó Copyright 2016 by the New England Botanical Club doi: 10.3119/16-07; first published on-line February 6, 2017. A BASELINE STUDY OF ALPINE SNOWBED AND RILL COMMUNITIES ON MOUNT WASHINGTON, NH ROBERT S. CAPERS Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269 e-mail: [email protected] NANCY G. SLACK Biology Department, The Sage Colleges, 65 1st St., Troy, NY 12180 ABSTRACT. -

Observations on Stem Surface Anatomy In
Arctoa (2000) 9: 151-154 OBSERVATIONS ON STEM SURFACE ANATOMY IN CLIMACIUM AND PLEUROZIOPSIS (CLIMACIACEAE: MUSCI) Î ÑÒÐÎÅÍÈÈ ÏÎÂÅÐÕÍÎÑÒÈ ÑÒÅÁËß CLIMACIUM È PLEUROZIOPSIS (CLIMACIACEAE: MUSCI) DANIEL H. NORRIS1 & MICHAEL S. IGNATOV2 ÄÀÍÈÝËÜ Ã. ÍÎÐÐÈÑ1 & ÌÈÕÀÈË Ñ. ÈÃÍÀÒÎÂ2 Abstract Pleuroziopsis was segregated from Climaciaceae into a separate monotypic family Pleuroziopsidaceae by Ireland (1968). According to Ireland, the most important and unique characteristic of Pleuroziopsis is a fluting of the stem by streaks of stem lamellae. However, this pattern of stem fluting is shown to be shared with the genus Climacium, so Pleuroziopsis is returned to Climaciaceae. Ðåçþìå Pleuroziopsis áûë âûäåëåí èç ñåìåéñòâà Climaciaceae â ñàìîñòîÿòåëüíîå ìîíîòèïíîå ñåìåéñòâî Pleuroziopsidaceae (Ireland, 1968). Îñíîâàíèåì äëÿ ýòîãî ïîñëóæèëî íàëè÷èå ïðîäîëüíûõ ‘ñòåáëåâûõ ïëàñòèí’, ñòðóêòóðû, êàê ñ÷èòàë Àéðåëàíä, íå èìåþùåé ãîìîëîãîâ. Îäíàêî, äàííîå èññëåäîâàíèå âûÿâèëî, ÷òî ñòðóêòóðà ïîâåðõíîñòè ñòåáëÿ ó Climacium è Pleuroziopsis ïðèíöèïèàëüíî ñõîäíà, òàê ÷òî ïîñëåäíèé ðîä ïðåäëàãàåòñÿ âåðíóòü â Climaciaceae. Many years ago, I (DHN) found myself with anatomy was first thought to allow easy sorting R. Ochyra, Krakow, in several very profitable dis- according to the observations of Ireland (1968). I cussions on paraphyllial arrangement in Hyp- (DHN) removed the leaves from both the stem nobryalean mosses. These discussions led in sub- and rhizome material of the two species. At that sequent years to careful observation of the pat- point, the cloaking of rhizoids was so dense as to terns of paraphyllial insertion of moss stems. A require plucking of those rhizoids with fine-point recent trip by DHN to Alaska afforded oppor- forceps. It was with great surprise that we found tunity to study freshly collected material from the denuded stems (split longitudinally to allow a population of Climacium dendroides (Hedw.) increased transparency) to be essentially identi- Web. -
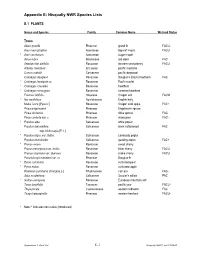
Appendix E Nisqually Species List
Appendix E: Nisqually NWR Species Lists E.1 PLANTS Genus and Species Family Common Name Wetland Status Trees Abies grandis Pinaceae grand fir FACU- Acer macrophyllum Aceraceae big-leaf maple FACU * Acer saccharum Aceraceae sugar maple Alnus rubra Betulaceae red alder FAC Amelanchier alnifolia Rosaceae western serviceberry FACU Arbutus menziesii Ericaceae pacific madrone Cornus nuttallii Cornaceae pacific dogwood Crataegus douglasii Rosaceae Douglas’s (black) hawthorn FAC * Crataegus laevigata cv. Rosaceae Paul's scarlet * Crataegus x lavallei Rosaceae hawthorn * Crataegus monogyna Rosaceae common hawthorn Fraxinus latifolia Oleaceae Oregon ash FACW * Ilex aquifolium Aquifoliaceae English holly Malus fusca [Pyrus f.] Rosaceae Oregon crab apple FAC+ Picea engelmannii Pinaceae Engelmann spruce Picea sitchensis Pinaceae Sitka spruce FAC Pinus contorta var. c. Pinaceae shore pine FAC- * Populus alba Salicaceae white poplar Populus balsamifera Salicaceae black cottonwood FAC ssp. trichocarpa [P. t. ] * Populus nigra var. italica Salicaceae Lombardy poplar Populus tremuloides Salicaceae quaking aspen FAC+ * Prunus avium Rosaceae sweet cherry Prunus emarginata var. mollis Rosaceae bitter cherry FACU Prunus virginiana var. demissa Rosaceae choke cherry FACU Pseudotsuga menziesii var. m. Pinaceae Douglas-fir * Pyrus communis Rosaceae cultivated pear * Pyrus malus Rosaceae cultivated apple Rhamnus purshiana [Frangula p.] Rhamnaceae cascara FAC- Salix scouleriana Salicaceae Scouler’s willow FAC * Sorbus aucuparia Rosaceae European mountain ash Taxus brevifolia Taxaceae pacific yew FACU- Thuja plicata Cupressaceae western redcedar FAC Tsuga heterophylla Pinaceae western hemlock FACU- * Note: * indicates non-native (introduced) Appendix E.1: Plant List E-1 Nisqually NWR Final CCP/EIS Genus and Species Family Common Name Wetland Status Shrubs, Brambles & Vines Acer circinatum Aceraceae vine maple FACU+ Arctostaphylos uva-ursi var. -

Spoon-Leaved Moss Bryoandersonia Illecebra
COSEWIC Assessment and Status Report on the Spoon-leaved Moss Bryoandersonia illecebra in Canada ENDANGERED 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION DES ENDANGERED WILDLIFE ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and status report on the spoon-leaved moss Bryoandersonia illecebra in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 31 pp. Doubt, J 2003. COSEWIC status report on the spoon-leaved moss Bryoandersonia illecebra in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-31 pp. Production note: COSEWIC would like to acknowledge Jennifer C. Doubt for writing the status report on the spoon-leaved moss Bryoandersonia illecebra, prepared under the contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur la situation de l’andersonie charmante (Bryoandersonia illecebra) au Canada Cover illustration: Spoon-leaved moss — Provided by the author. Her Majesty the Queen in Right of Canada 2003 Catalogue No. CW69-14/331-2003E-PDF ISBN 0-662-35350-1 HTML: CW69-14/331-2003E-HTML 0-662-35351-X Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2003 Common name Spoon-leaved moss Scientific name Bryoandersonia illecebra Status Endangered Reason for designation This species is endemic to eastern North America. -

Biodiversity
Appendix I Biodiversity Appendix I1 Literature Review – Biodiversity Resources in the Oil Sands Region of Alberta Syncrude Canada Ltd. Mildred Lake Extension Project Volume 3 – EIA Appendices December 2014 APPENDIX I1: LITERATURE REVIEW – BIODIVERSITY RESOURCES IN THE OIL SANDS REGION OF ALBERTA TABLE OF CONTENTS PAGE 1.0 BIOTIC DIVERSTY DATA AND SUMMARIES ................................................................ 1 1.1 Definition ............................................................................................................... 1 1.2 Biodiversity Policy and Assessments .................................................................... 1 1.3 Environmental Setting ........................................................................................... 2 1.3.1 Ecosystems ........................................................................................... 2 1.3.2 Biota ...................................................................................................... 7 1.4 Key Issues ............................................................................................................. 9 1.4.1 Alteration of Landscapes and Landforms ............................................. 9 1.4.2 Ecosystem (Habitat) Alteration ........................................................... 10 1.4.3 Habitat Fragmentation and Edge Effects ............................................ 10 1.4.4 Cumulative Effects .............................................................................. 12 1.4.5 Climate Change ................................................................................. -

Allenberg Bog Plant List
ALLENBERG BOG AUDUBON NATURE PRESERVE Allenberg Bog is also known to some as Waterman's Swamp, Congdon's Pond, and Owlenburg Bog and is on the border of the towns of Napoli and New Albion, New York in Cattaraugus County. A unique and fascinating refuge of 390 acres, it is the jewel of the Buffalo Audubon Preserve System. Even before the first parcels joined Audubon's preserve holdings in 1957, the area was famous among botanists for its wild orchids, more than 30 species of liverworts, nearly 60 species of mosses, and approximately 258 species of vascular plants. It should be noted that any collecting of any plants in this or any of our refuges is strictly prohibited. Please respect the purposes behind "Preserves." Plant List: Liverworts Family Ptilidiaceae Trichocolea tomentella Family Lepidoziaceae Bazzania trilobata Family Calypogeiaceae Calypogeia neesiana Calypogeia sphagnicola Calypogeia trichomanis Family Cephaloziaceae Cephalozia connivens Cephalozia media Cladopodiella fluitans Family Jungermanniaceae Lophoxia gracilis Jamesoniella autumnalis Plectocolea crenulata Family Harpanthaceae Lophocolea heterophylla Chiloscyphus pallescens Harpanthus scutatus Geocalyx graveolans Family Porellaceae Porella platyphylloides Family Radulaceae Radula complanata Family Frullaniaceae Frullania asagrayana Frullania brittoniae Frullania eboracensis Frullania oakesiana Frullania tamirisci Family Pelliaceae Pellia jabbroniana Family Pallavicniaceae Pallavicinia lyelli Family Riccardiaceae Riccardia latrifons Riccardia multifida Family Marchantiaceae -

Article Antiproliferative and Antimicrobial Activities of Selected Bryophytes Martin Vollár1
Article Antiproliferative and Antimicrobial Activities of Selected Bryophytes Martin Vollár 1,2, András Gyovai 3, Péter Szűcs 4, István Zupkó 3, Marianna Marschall 4, Boglárka Csupor-Löffler 1,2, Péter Bérdi 3, Anikó Vecsernyés 1, Attila Csorba 1, Erika Liktor-Busa 1, Edit Urbán 5 and Dezső Csupor 1,2,* 1 Department of Pharmacognosy, Faculty of Pharmacy, University of Szeged, H-6720 Szeged, Hungary; [email protected] (M.V.); [email protected] (B.C.-L.); [email protected] (A.V.); [email protected] (A.C.); [email protected] (E.L.-B.) 2 Interdisciplinary Centre for Natural Products, University of Szeged, H-6720 Szeged, Hungary 3 Department of Pharmacodynamics and Biopharmacy, Faculty of Pharmacy, University of Szeged, H-6720 Szeged, Hungary; [email protected] (A.G.); [email protected] (I.Z.); [email protected] (P.B.) 4 Department of Botany and Plant Physiology, Institute of Biology, Eszterházy Károly University, H-3300 Eger, Hungary; [email protected] (P.S.); [email protected] (M.M.) 5 Institute of Clinical Microbiology, Faculty of Medicine, University of Szeged, H-6720 Szeged, Hungary; [email protected] * Correspondence: [email protected]; Tel.: +36-62-545-559 Academic editor: Zhe-Sheng (Jason) Chen and Dong-Hua Yang Received: 28 May 2018; Accepted: 20 June 2018; Published: 23 June 2018 Abstract: One-hundred and sixty-eight aqueous and organic extracts of 42 selected bryophyte species were screened in vitro for antiproliferative activity on a panel of human gynecological cancer cell lines containing HeLa (cervix epithelial adenocarcinoma), A2780 (ovarian carcinoma), and T47D (invasive ductal breast carcinoma) cells using the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay and for antibacterial activity on 11 strains using the disc- diffusion method. -

PRZYRODA SUDETÓW Tom 16 2013
2013 Tom 16 2013 1 – Pluszcz Cinclus cinclus (fot. K. Żarkowski) 2 – Czyż Carduelis spinus (fot. W. Bena) 3 – Orzechówka Nucifraga caryocatactes (fot. K. Żarkowski) 4 – Cietrzew Tetrao tetrix (fot. M. Martini) Tom 16 1 2 3 4 SUDETÓW PRZYRODA Publikacja dofinansowana ze środków Wojewódzkiego Funduszu Ochrony Środowiska i Gospodarki Wodnej we Wrocławiu Sóweczka Glaucidium passerinum w Sudetach Zachodnich w 2009 r. (fot. K. Dobro- wolska-Martini). MUZEUM PRZYRODNICZE w JELENIEJ GÓRZE ZACHODNIOSUDECKIE TOWARZYSTWO PRZYRODNICZE PRZYRODA SUDETÓW ROCZNIK Tom 16, 2013 Naturam si sequemur ducem, nunquam aberrabimus JELENIA GÓRA 2013 Redaktor naukowy BOŻENA GRAMSZ Zespó³ redakcyjny BO¯ENA GRAMSZ CZES£AW NARKIEWICZ STANISŁAW FIRSZT Recenzenci ADAM BORATYńSKI (Kórnik) ADAM MALKIEWICZ (Wrocław) ZYGMUNT DAJDOK (Wrocław) ANNA RONIKIER (Kraków) EWA FUDALI (Wrocław) DARIUSZ SKARŻYńSKI (Wrocław) ANNA JAKUBSKA-BUSSE (Wrocław) EWA SZCZęŚNIAK (Wrocław) LESZEK JERZAK (Zielona Góra) MICHAŁ ŚLIWIńSKI (Wrocław) ZYGMUNT KąCKI (Wrocław) DARIUSZ TARNAWSKI (Wrocław) ALICJA KRZEMIńSKA (Wrocław) BARBARA TOKARSKA-GUZIK (Katowice) AGNIESZKA LATOCHA (Wrocław) ANDRZEJ WARCHAŁOWSKI (Wrocław) LUDWIK LIPNICKI (Gorzów Wielkopolski) BRONISŁAW WOJTUń (Wrocław) T³umaczenie streszczeñ BARTOSZ BORCZYK Konsultacje językowe BEATA POKRYSZKO Dtp „AD REM”, tel. 75 75 222 15, www.adrem.jgora.pl Oprac. kartograficzne „PLAN”, tel. 75 75 260 77 (str. 8, 43, 76) Druk Leyko, Kraków Nak³ad 1200 egz. Wydawca MUZEUM PRZYRODNICZE w JELENIEJ GÓRZE oraz ZACHODNIOSUDECKIE TO WA RZY STWO PRZYRODNICZE Adres redakcji: 58-560 Jelenia Góra, ul. Wolnoœci 268 tel./fax 75 75 515 06 e-mail: [email protected] e-mail: [email protected] www.muzeum-cieplice.pl ISSN 1895-8109 Na okładce: Kwisa powyżej Świeradowa-Zdroju (fot. W. Bena). PRZYRODA SUDETÓW t. -

An Updated List of Mosses of Korea
Journal of Species Research 9(4):377-412, 2020 An updated list of mosses of Korea Wonhee Kim1,*, Masanobu Higuchi2 and Tomio Yamaguchi3 1National Institute of Biological Resources, 42 Hwangyeong-ro, Seo-gu, Incheon 22689 Republic of Korea 2Department of Botany, National Museum of Nature and Science, 4-1-1 Amakubo, Tsukuba 305-0005 Japan 3Program of Basci Biology, Graduate School of Integrated Science for Life, Hiroshima University, 1-3-1 Kagamiyama, Higashi-hiroshima-shi 739-8526 Japan *Correspondent: [email protected] Cardot (1904) first reported 98 Korean mosses, which were collected from Busan, Gangwon Province, Mokpo, Seoul, Wonsan and Pyongyang by Father Faurie in 1901. Thirty-four of these species were new species to the world. However, eight of these species have been not listed to the moss checklist of Korea before this study. Thus, this study complies the literature including Korean mosses, and lists all the species there. As the result, the moss list of Korea is updated as including 775 taxa (728 species, 7 subspecies, 38 varieties, 2 forma) arranged into 56 families and 250 genera. This list include species that have been newly recorded since 1980. Brachythecium is the largest genus in Korea, and Fissidens, Sphagnum, Dicranum and Entodon are relatively large. Additionally, this study cites specimens collected from Jeju Island, Samcheok, Gangwon Province, and Socheong Island, and it is possible to confirm the distribution of 338 species in Korea. Keywords: bryophytes, checklist, Korea, mosses, updated Ⓒ 2020 National Institute of Biological Resources DOI:10.12651/JSR.2020.9.4.377 INTRODUCTION Choi (1980), Park and Choi (2007) reported a “New List of Bryophytes in Korea” by presenting an overview of The first study on Korean bryophytes was published by bryophytes surveyed in Mt.