Cross-Modal Mechanisms: Perceptual Multistability in Audition and Vision
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review and Selective Analysis of 3D Display Technologies for Anatomical Education
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2018 A Review and Selective Analysis of 3D Display Technologies for Anatomical Education Matthew Hackett University of Central Florida Part of the Anatomy Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Hackett, Matthew, "A Review and Selective Analysis of 3D Display Technologies for Anatomical Education" (2018). Electronic Theses and Dissertations, 2004-2019. 6408. https://stars.library.ucf.edu/etd/6408 A REVIEW AND SELECTIVE ANALYSIS OF 3D DISPLAY TECHNOLOGIES FOR ANATOMICAL EDUCATION by: MATTHEW G. HACKETT BSE University of Central Florida 2007, MSE University of Florida 2009, MS University of Central Florida 2012 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Modeling and Simulation program in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2018 Major Professor: Michael Proctor ©2018 Matthew Hackett ii ABSTRACT The study of anatomy is complex and difficult for students in both graduate and undergraduate education. Researchers have attempted to improve anatomical education with the inclusion of three-dimensional visualization, with the prevailing finding that 3D is beneficial to students. However, there is limited research on the relative efficacy of different 3D modalities, including monoscopic, stereoscopic, and autostereoscopic displays. -

Symmetric Networks with Geometric Constraints As Models of Visual Illusions
S S symmetry Article Symmetric Networks with Geometric Constraints as Models of Visual Illusions Ian Stewart 1,*,† and Martin Golubitsky 2,† 1 Mathematics Institute, University of Warwick, Coventry CV4 7AL, UK 2 Department of Mathematics, Ohio State University, Columbus, OH 43210, USA; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Received: 17 May 2019; Accepted: 13 June 2019; Published: 16 June 2019 Abstract: Multistable illusions occur when the visual system interprets the same image in two different ways. We model illusions using dynamic systems based on Wilson networks, which detect combinations of levels of attributes of the image. In most examples presented here, the network has symmetry, which is vital to the analysis of the dynamics. We assume that the visual system has previously learned that certain combinations are geometrically consistent or inconsistent, and model this knowledge by adding suitable excitatory and inhibitory connections between attribute levels. We first discuss 4-node networks for the Necker cube and the rabbit/duck illusion. The main results analyze a more elaborate model for the Necker cube, a 16-node Wilson network whose nodes represent alternative orientations of specific segments of the image. Symmetric Hopf bifurcation is used to show that a small list of natural local geometric consistency conditions leads to alternation between two global percepts: cubes in two different orientations. The model also predicts brief transitional states in which the percept involves impossible rectangles analogous to the Penrose triangle. A tristable illusion generalizing the Necker cube is modelled in a similar manner. -

A Review and Selective Analysis of 3D Display Technologies for Anatomical Education
A REVIEW AND SELECTIVE ANALYSIS OF 3D DISPLAY TECHNOLOGIES FOR ANATOMICAL EDUCATION by: MATTHEW G. HACKETT BSE University of Central Florida 2007, MSE University of Florida 2009, MS University of Central Florida 2012 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Modeling and Simulation program in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Summer Term 2018 Major Professor: Michael Proctor ©2018 Matthew Hackett ii ABSTRACT The study of anatomy is complex and difficult for students in both graduate and undergraduate education. Researchers have attempted to improve anatomical education with the inclusion of three-dimensional visualization, with the prevailing finding that 3D is beneficial to students. However, there is limited research on the relative efficacy of different 3D modalities, including monoscopic, stereoscopic, and autostereoscopic displays. This study analyzes educational performance, confidence, cognitive load, visual-spatial ability, and technology acceptance in participants using autostereoscopic 3D visualization (holograms), monoscopic 3D visualization (3DPDFs), and a control visualization (2D printed images). Participants were randomized into three treatment groups: holograms (n=60), 3DPDFs (n=60), and printed images (n=59). Participants completed a pre-test followed by a self-study period using the treatment visualization. Immediately following the study period, participants completed the NASA TLX cognitive load instrument, a technology acceptance instrument, visual-spatial ability instruments, a confidence instrument, and a post-test. Post-test results showed the hologram treatment group (Mdn=80.0) performed significantly better than both 3DPDF (Mdn=66.7, p=.008) and printed images (Mdn=66.7, p=.007). -

Hemifield-Specific Rotational Biases During the Observation Of
S S symmetry Article Hemifield-Specific Rotational Biases during the Observation of Ambiguous Human Silhouettes Chiara Lucafò *,† , Daniele Marzoli *,†, Caterina Padulo , Stefano Troiano, Lucia Pelosi Zazzerini, Gianluca Malatesta , Ilaria Amodeo and Luca Tommasi Department of Psychological Sciences, Health and Territory, University of Chieti, Via dei Vestini 29, I-66013 Chieti, Italy; [email protected] (C.P.); [email protected] (S.T.); [email protected] (L.P.Z.); [email protected] (G.M.); [email protected] (I.A.); [email protected] (L.T.) * Correspondence: [email protected] (C.L.); [email protected] (D.M.) † These authors equally contributed to this work. Abstract: Both static and dynamic ambiguous stimuli representing human bodies that perform unimanual or unipedal movements are usually interpreted as right-limbed rather than left-limbed, suggesting that human observers attend to the right side of others more than the left one. Moreover, such a bias is stronger when static human silhouettes are presented in the RVF (right visual field) than in the LVF (left visual field), which might represent a particular instance of embodiment. On the other hand, hemispheric-specific rotational biases, combined with the well-known bias to perceive forward-facing figures, could represent a confounding factor when accounting for such findings. Therefore, we investigated whether the lateralized presentation of an ambiguous rotating human body would affect its perceived handedness/footedness (implying a role of motor representations), Citation: Lucafò, C.; Marzoli, D.; its perceived spinning direction (implying a role of visual representations), or both. To this aim, we Padulo, C.; Troiano, S.; required participants to indicate the perceived spinning direction (which also unveils the perceived Pelosi Zazzerini, L.; Malatesta, G.; handedness/footedness) of ambiguous stimuli depicting humans with an arm or a leg outstretched. -

50 Optical Illusions Free
FREE 50 OPTICAL ILLUSIONS PDF Sam Taplin | none | 30 Oct 2009 | Usborne Publishing Ltd | 9781409507796 | English | London, United Kingdom “50 optical illusions” in Usborne Quicklinks Optical illusions are presentations of objects 50 Optical Illusions well as situations in such a 50 Optical Illusions, that it confuses your vision, and projects double meaning of the same image to you simultaneously. Optical illusions are really fun to see, as they confuse 50 Optical Illusions about what the picture really is, and play with our minds. So, for you to enjoy seeing some optical illusions and have your mind really confused, here is a list of some superb and mind-blowing optical illusions that will surely leave you confused and wondering, figuring out the real situation in the images. This article is also categorized into 50 Optical Illusions sections — Photographic Illusions, consisting of 50 Optical Illusions illusions in a taken photo of any situation, and Graphical Illusions, containing computer generated or hand-drawn illusions. Very Interesting Object. Pyramid Block. Realistic Dice Illusion. Green Pouch. Chess Illusion. Intersecting Buildings. Courtyard or Terrace? Half here, Half there. Bathroom Mirror Illusion. Which one is Cut: Wood or the Metal. Pressed In or Out? Truck Painting Illusion. Oh No! The Rug is Sinking! That Sinking Feeling. Crazy Car Optical Illusion. Are the Blue Lines horizontal? Impossible Arch. Move for You. Wooden Box Illusion. Moving Circles Illusion. Colourful Object Illusion. See beyond the Skull Illusion. Corner House Illusion. Moving Waves. Moving Bicycle Wheels. Two Faces or Two People? Staring at the Dot makes Grey Disappear. Old Man Illusion. -

Book XVII License and the Law Editor: Ramon F
8 88 8 8nd 8 8888on.com 8888 Basic Photography in 180 Days Book XVII License and the Law Editor: Ramon F. aeroramon.com Contents 1 Day 1 1 1.1 Photography and the law ....................................... 1 1.1.1 United Kingdom ....................................... 2 1.1.2 United States ......................................... 6 1.1.3 Hong Kong .......................................... 8 1.1.4 Hungary ............................................ 8 1.1.5 Macau ............................................. 8 1.1.6 South Africa ......................................... 8 1.1.7 Sudan and South Sudan .................................... 9 1.1.8 India .............................................. 10 1.1.9 Iceland ............................................ 10 1.1.10 Spain ............................................. 10 1.1.11 Mexico ............................................ 10 1.1.12 See also ............................................ 10 1.1.13 Notes ............................................. 10 1.1.14 References .......................................... 10 1.1.15 External links ......................................... 12 2 Day 2 13 2.1 Observation .............................................. 13 2.1.1 Observation in science .................................... 14 2.1.2 Observational paradoxes ................................... 14 2.1.3 Biases ............................................. 15 2.1.4 Observations in philosophy .................................. 16 2.1.5 See also ........................................... -
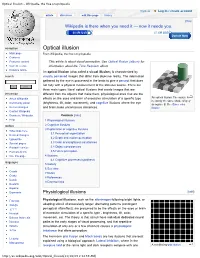
Optical Illusion - Wikipedia, the Free Encyclopedia
Optical illusion - Wikipedia, the free encyclopedia Try Beta Log in / create account article discussion edit this page history [Hide] Wikipedia is there when you need it — now it needs you. $0.6M USD $7.5M USD Donate Now navigation Optical illusion Main page From Wikipedia, the free encyclopedia Contents Featured content This article is about visual perception. See Optical Illusion (album) for Current events information about the Time Requiem album. Random article An optical illusion (also called a visual illusion) is characterized by search visually perceived images that differ from objective reality. The information gathered by the eye is processed in the brain to give a percept that does not tally with a physical measurement of the stimulus source. There are three main types: literal optical illusions that create images that are interaction different from the objects that make them, physiological ones that are the An optical illusion. The square A About Wikipedia effects on the eyes and brain of excessive stimulation of a specific type is exactly the same shade of grey Community portal (brightness, tilt, color, movement), and cognitive illusions where the eye as square B. See Same color Recent changes and brain make unconscious inferences. illusion Contact Wikipedia Donate to Wikipedia Contents [hide] Help 1 Physiological illusions toolbox 2 Cognitive illusions 3 Explanation of cognitive illusions What links here 3.1 Perceptual organization Related changes 3.2 Depth and motion perception Upload file Special pages 3.3 Color and brightness -

Chapter 6 Visual Perception
Chapter 6 Visual Perception Steven M. LaValle University of Oulu Copyright Steven M. LaValle 2019 Available for downloading at http://vr.cs.uiuc.edu/ 154 S. M. LaValle: Virtual Reality Chapter 6 Visual Perception This chapter continues where Chapter 5 left off by transitioning from the phys- iology of human vision to perception. If we were computers, then this transition might seem like going from low-level hardware to higher-level software and algo- rithms. How do our brains interpret the world around us so effectively in spite of our limited biological hardware? To understand how we may be fooled by visual stimuli presented by a display, you must first understand how our we perceive or interpret the real world under normal circumstances. It is not always clear what we will perceive. We have already seen several optical illusions. VR itself can be Figure 6.1: This painting uses a monocular depth cue called a texture gradient to considered as a grand optical illusion. Under what conditions will it succeed or enhance depth perception: The bricks become smaller and thinner as the depth fail? increases. Other cues arise from perspective projection, including height in the vi- Section 6.1 covers perception of the distance of objects from our eyes, which sual field and retinal image size. (“Paris Street, Rainy Day,” Gustave Caillebotte, is also related to the perception of object scale. Section 6.2 explains how we 1877. Art Institute of Chicago.) perceive motion. An important part of this is the illusion of motion that we perceive from videos, which are merely a sequence of pictures. -

Dissertation Title Page Template
University of Nevada, Reno TOP-DOWN MODULATION OF THE CHROMATIC VEP WITH HYPNOTIC SUGGESTION A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Psychology by Chad Steven Duncan Dr. Michael A. Crognale / Dissertation Advisor May 2013 THE GRADUATE SCHOOL We recommend that the dissertation prepared under our supervision by CHAD S. DUNCAN entitled Top-down Modulation of the Chromatic VEP with Hypnotic Suggestion be accepted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Michael A. Crognale, Ph.D., Advisor Gideon P. Caplovitz, Ph.D., Committee Member Michael A. Webster, Ph.D., Committee Member William Danton, Ph.D., Committee Member Thomas J. Nickles, Ph.D., Graduate School Representative Marsha H. Read, Ph. D., Dean, Graduate School May, 2013 i Abstract Visual perception is composed of both bottom-up (stimulus driven) and top-down (feedback) processes. It has been demonstrated that directed spatial attention enhances processing of attended stimuli, inhibits non-attended stimuli, and increases baseline activity in portions of cortex corresponding to attended areas of the visual scene in the absence of a stimulus. However, mechanisms of attention appear to influence processing of chromatic stimuli differently than that of achromatic stimuli. Visual evoked potentials (VEPs) recorded under such conditions agree with this pattern of activation when elicited by purely achromatic grating stimuli. However, when stimuli were chosen to preferentially activate the S-(L+M) or L-M chromatically opponent pathways, no changes in signal were detected as a function of attention, suggesting different amounts of feedback, or different mechanisms of feedback, reaching the visual cortex where the VEP is thought to originate. -
Distal Attribution in Sensory Substitution
SPACING OUT: DISTAL ATTRIBUTION IN SENSORY SUBSTITUTION ____________________________________ A Thesis Presented to The Honors Tutorial College Ohio University _______________________________________ In Partial Fulfillment of the Requirements for Graduation from the Honors Tutorial College with the degree of Bachelor of Arts in Philosophy ______________________________________ by David E. Pence May 2013 Contents 1. Introduction: The ABCs of SSDs 1 1.1 More Background: Sensory Substitution and Enactivism 4 2. Why Action May not be Necessary 10 2.1 Seeing Without Structure 13 2.2 Seeing Without Corroboration? 18 2.3 A Thought Experiment 22 3. Alternative Frameworks for Sensory Substitution 25 3.1 The Prospects Considered 32 3.2. How it’s Done 32 3.3 Where it Happens 37 4. Crossmodal Plasticity 40 4.1 The Mechanisms of Plasticity 41 4.2 Where the Senses Trade 46 4.3 Acquired Synaesthesia? 48 5. The Multimodal Mind 52 5.1 Where it All Comes Together 53 5.2 Integration at Work 57 5.3 A Second Multimodal Proposal 67 6. Mental Imagery 71 6.1 The Step-by-Step 72 6.2 The Land of Imagination 77 6.3 Speaking For and Against 79 6.4 Does Reading Make a Better Metaphor? 87 7. How They All Fit Together 93 8. Conclusions: Lessons and Future Possibilities 97 9. References 100 List of Figures Figure 1: Retinal Disparity 9 Figure 2: Gibsonian Ecological Perception 9 Figure 3: High Contrast Image 14 Figure 4: Resolution of Early TVSS 16 Figure 5: Early TVSS Device 19 Figure 6: Working Memory Map 34 Figure 7: Map of Brain Regions Involved in Sensory Substitution 39 Figure 8: Multisensory Reverse Hierarchy Theory 69 Figure 9: Resolution of The vOICe 89 1 1. -

Stroop Tasks with Visual and Auditory Stimuli
Stroop tasks with visual and auditory stimuli How different combinations of spoken words, written words, images and natural sounds affect reaction times Christina Malapetsa Department of Linguistics Bachelor Thesis 15HP Psycho/Neurolinguistics Experimental linguistics 15HP Spring term 2020 Supervisors: Pétur Helgason, Marcin Włodarczak Stroop tasks with visual and auditory stimuli How different combinations of spoken words, written words, images and natural sounds affect reaction times Christina Malapetsa Abstract The Stroop effect is the delay in reaction times due to interference. Since the original experiments of 1935, it has been used primarily in linguistic context. Language is a complex skill unique to humans, which involves a large part of the cerebral cortex and many subcortical regions. It is perceived primarily in auditory form (spoken) and secondarily in visual form (written), but it is also always perceived in representational form (natural sounds, images, smells, etc). Auditory signals are processed much faster than visual signals, and the language processing centres are closer to the primary auditory cortex than the primary visual cortex, but due to the integration of stimuli and the role of the executive functions, we are able to perceive both simultaneously and coherently. However, auditory signals are still processed faster, and this study focused on establishing how auditory and visual, linguistic and representational stimuli interact with each other and affect reaction times in four Stroop tasks with four archetypal mammals (dog, cat, mouse and pig): a written word against an image, a spoken word against an image, a written word against a natural sound and a spoken word against a natural sound. -
Copyright and Use of This Thesis This Thesis Must Be Used in Accordance with the Provisions of the Copyright Act 1968
COPYRIGHT AND USE OF THIS THESIS This thesis must be used in accordance with the provisions of the Copyright Act 1968. Reproduction of material protected by copyright may be an infringement of copyright and copyright owners may be entitled to take legal action against persons who infringe their copyright. Section 51 (2) of the Copyright Act permits an authorized officer of a university library or archives to provide a copy (by communication or otherwise) of an unpublished thesis kept in the library or archives, to a person who satisfies the authorized officer that he or she requires the reproduction for the purposes of research or study. The Copyright Act grants the creator of a work a number of moral rights, specifically the right of attribution, the right against false attribution and the right of integrity. You may infringe the author’s moral rights if you: - fail to acknowledge the author of this thesis if you quote sections from the work - attribute this thesis to another author - subject this thesis to derogatory treatment which may prejudice the author’s reputation For further information contact the University’s Director of Copyright Services sydney.edu.au/copyright A cross-modal investigation into the relationship between bistable perception and a global temporal mechanism by Amanda Louise Parker A thesis submitted in total fulfilment of the requirements of a Doctor of Philosophy in Science School of Psychology The University of Sydney 2013 Table of contents ORGANISATION OF THE THESIS ........................................................................................................