Masteroppgave (4.517Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Søknad Solhom
Konsesjonssøknad Solhom - Arendal Spenningsoppgradering Søknad om konsesjon for ombygging fra 300 til 420 kV 1 Desember 2012 Forord Statnett SF legger med dette frem søknad om konsesjon, ekspropriasjonstillatelse og forhånds- tiltredelse for spenningsoppgradering (ombygging) av eksisterende 300 kV-ledning fra Arendal transformatorstasjon i Froland kommune til Solhom kraftstasjon i Kvinesdal kommune. Ledningen vil etter ombyggingen kunne drives med 420 kV spenning. Spenningsoppgraderingen og tilhørende anlegg vil berøre Froland, Birkenes og Evje og Hornnes kommuner i Aust-Agder og Åseral og Kvinesdal kommuner i Vest-Agder. Oppgraderingen av 300 kV- ledningen til 420 kV på strekningen Solhom – Arendal er en del av det større prosjektet ”Spennings- oppgraderinger i Vestre korridor” og skal legge til rett for sikker drift av nettet på Sørlandet, ny fornybar kraftproduksjon, fri utnyttelse av kapasiteten på nye og eksisterende mellomlandsforbindelser og fleksibilitet for fremtidig utvikling. Konsesjonssøknaden oversendes Norges vassdrags- og energidirektorat (NVE) til behandling. Høringsuttalelser sendes til: Norges vassdrags- og energidirektorat Postboks 5091, Majorstuen 0301 OSLO e-post: [email protected] Saksbehandler: Kristian Marcussen, tlf: 22 95 91 86 Spørsmål vedrørende søknad kan rettes til: Funksjon/stilling Navn Tlf. nr. Mobil e-post Delprosjektleder Tor Morten Sneve 23903015 40065033 [email protected] Grunneierkontakt Ole Øystein Lunde 23904121 99044815 [email protected] Relevante dokumenter og informasjon om prosjektet og Statnett finnes på Internettadressen: http://www.statnett.no/no/Prosjekter/Vestre-korridor/Solhom---Arendal/ Oslo, desember 2012 Håkon Borgen Konserndirektør Divisjon Nettutbygging 2 Sammendrag Statnett er i gang med å bygge neste generasjon sentralnett. Dette vil bedre forsyningssikkerheten og øke kapasiteten i nettet, slik at det legges til rette for mer klimavennlige løsninger og økt verdiskaping for brukerne av kraftnettet. -

NGU Norges Geologiske Undersøkelse Geolological Survey of Norway
NGU Norges geologiske undersøkelse Geolological Survey of Norway Bulletin 440 MISCELLANEOUS RESEARCH PAPERS Trondheim 2002 Printed in Norway by Grytting AS Contents Timing of late- to post-tectonic Sveconorwegian granitic magmatism in South Norway .............................................................................................. 5 TOM ANDERSEN, ARILD ANDRESEN & ARTHUR G. SYLVESTER Age and petrogenesis of the Tinn granite,Telemark, South Norway, and its geochemical relationship to metarhyolite of the Rjukan Group ............................. 19 TOM ANDERSEN, ARTHUR G. SYLVESTER & ARILD ANDRESEN Devonian ages from 40Ar/39Ar dating of plagioclase in dolerite dykes, eastern Varanger Peninsula, North Norway ......................................................................... 27 PHILIP G. GUISE / DAVID ROBERTS Mid and Late Weichselian, ice-sheet fluctuations northwest of the Svartisen glacier, Nordland, northern Norway ................................................................................. 39 LARS OLSEN Instructions to authors – NGU Bulletin .................................................................... xx TOM ANDERSEN,ARILD ANDRESEN & ARTHUR G.SYLVESTER NGU-BULL 440, 2002 - PAGE 5 Timing of late- to post-tectonic Sveconorwegian granitic magmatism in South Norway TOM ANDERSEN, ARILD ANDRESEN & ARTHUR G. SYLVESTER Andersen, T., Andresen, A. & Sylvester, A.G. 2002: Timing of late- to post-tectonic Sveconorwegian granitic magma- tism in South Norway. Norges geologiske undersøkelse Bulletin 440, 5-18. Dating of late- to post-tectonic Sveconorwegian granitic intrusions from South Norway by the SIMS U-Pb method on zircons and by internal Pb-Pb isochrons on rock-forming minerals indicates a major event of granitic magmatism all across southern Norway in the period 950 to 920 Ma. This magmatic event included emplacement of mantle- derived magma into the source region of granitic magmas in the lower crust east of the Mandal-Ustaoset shear zone, and formation of hybrid magmas containing crustal and mantle-derived components. -
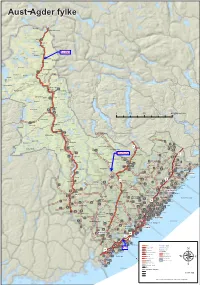
Aust−Agder Fylke
Aust−Agder fylke torhellerfjelli loros fjåen2fjellstove eggine pørsvssE fjelli freiveQRI enevssheii rovden Hartevatnet jørnrotu teinheii Vatndals− letteE vatnet tndlsE skurven dmmene Store Urevatn ferdlen Bykle ferdlsu roslemo fygdeheii Bykleheiane fyklestøyln QRP tvnes rovtn hytt Botsvatn fykle torvssE teinheii egnådlsE torsteinen heii Otra ygnestd QQT otemo uringlevtn qmsø euråhorten juven romme Raudvatnet Valleheiane tvskrdE hytt QQI lle vjomsnuten rddlsE fossu rolteheii Valle ppstd vrtenut QQR QQQ frokkeQQP ykstjørnheii rylestd 0204010 Kilometers ysstd rdvssheii wyklestøyl QQU øtefjell Øyuvsu festelnd eustd Hovatn einshornE W vngeid QPR wjåvsshytt heii qrnheim eiskvæven qjevden tkkedlen ustfjell eustd olhomfjell heii yse korv qukhei Gjøv UI ndnesÅrksø uongsfjell Bygland torrfjellet US VH ndnes qrunnetjørnsu Måvatn RI unndlsE Gjerstad ød UQ tosephsu QPQ freiung UR heii qjøvdlesklnd kåmedl hlePUP RIV QPP wosvld estøl våssen ØsterholtiIV rmreheii eustenå rdehei torlihei ylnd ndån WR QHR fyglnd Nidelva qryting romdrom wo RIU ovdl krsvssu Åmli PUI Gjavnes− UV WI undet Byglands− moen pine T vuvdl Øvre ullingsE PUU WI PUU sndre U rødneø fjorden hei PUS wjåvtn øndeled PUV ÅmliPUR egårshei Tovdalselva vuveik WQ kjeggedl festelihei RIT S QSI vongerk RIS R ivik Vegårshei F wyr IH P Barmen V WP eklnd RIT ÅrdlQPI kliknuten ippelnd fsvtn RIR Q woen isør RII W fås hølemo I qrendi RIQ xonnut øyslnd rovde ndnes Risør RIP iIV frumoen IP pie W RI ehus IHS vget fyglndsE orehei IHI tne ITR WS xipe temhei fjord vuvrk keidmo xelug PUQ IIQ RII QHP qutestdrimmelsyn -

Forekomst Av Reproduserende Bestander Av Bekke- Røye (Salvelinus Fontinalis) I Norge Pr
Forekomst av reproduserende bestander av bekke- røye (Salvelinus fontinalis) i Norge pr. 2013 Trygve Hesthagen og Einar Kleiven NINAs publikasjoner NINA Rapport Dette er en elektronisk serie fra 2005 som erstatter de tidligere seriene NINA Fagrapport, NINA Oppdragsmelding og NINA Project Report. Normalt er dette NINAs rapportering til oppdragsgiver etter gjennomført forsknings-, overvåkings- eller utredningsarbeid. I tillegg vil serien favne mye av instituttets øvrige rapportering, for eksempel fra seminarer og konferanser, resultater av eget forsk- nings- og utredningsarbeid og litteraturstudier. NINA Rapport kan også utgis på annet språk når det er hensiktsmessig. NINA Temahefte Som navnet angir behandler temaheftene spesielle emner. Heftene utarbeides etter behov og se- rien favner svært vidt; fra systematiske bestemmelsesnøkler til informasjon om viktige problemstil- linger i samfunnet. NINA Temahefte gis vanligvis en populærvitenskapelig form med mer vekt på illustrasjoner enn NINA Rapport. NINA Fakta Faktaarkene har som mål å gjøre NINAs forskningsresultater raskt og enkelt tilgjengelig for et større publikum. De sendes til presse, ideelle organisasjoner, naturforvaltningen på ulike nivå, politikere og andre spesielt interesserte. Faktaarkene gir en kort framstilling av noen av våre viktigste forsk- ningstema. Annen publisering I tillegg til rapporteringen i NINAs egne serier publiserer instituttets ansatte en stor del av sine viten- skapelige resultater i internasjonale journaler, populærfaglige bøker og tidsskrifter. Forekomst -

Project Description and Program
LINJELANGS ´along the line´ BERGEN - STAVANGER DOVREBANEN RØROSBANEN TABLE OF CONTENTS Connecting 6 villages along Sørlandsbanen.......................................... 5 HAMAR 01:00h 29 500 inhabitants Studytrip stop no. 1 BERGENSBANEN Railroad as an urbanism...................................... 7 BERGEN 08:00h 255 464 inhabitants Studytrip start The rural Experience .......................................... 9 Community railroad ............................................ 11 00:50h Nelaug................................................................ 13 OSLO 00:40h 1 000 500 inhabitants 00:45h Studytrip intersection point Linjelangs intervention........................................ 15 MOSS Participants........................................................ 17 31 000 inhabitants 00:55h Studytrip stop no. 2 PORSGRUNN 35 500 inhabitants Studytrip stop no. 3 STAVANGER 222 697 inhabitants Studytrip end 00:40h NELAUG 150 inhabitants THE SOUTHERN RAILWAY ARENDAL 44 643 inhabitants 07:45h Closed station Kristiansand 85 983 inhabitants Cities/Comunity hubs Airport 0 km 500 km CONNECTING 6 VILLAGES ALONG SØRLANDSBANEN This project aims to strengthen and revital- ize 6 rural villages along the Southern rail- road in Norway, Sørlandsbanen. To achieve this, we have constructed a two parted re- gional strategy. The first part of the strategy is strength- ening and using the existing railroad as a connection between the 6 villages. This is possible by providing a local train that op- erates between the villages. The second part is using architectural in- tervention to enhance the local resources and strengthen the connection between the village and the railroad. This strategy is based on the paradigm; that a single village is not big enough to sustain a fundamental program, but together they are big enough to function as a small scale city, with the possibility to sustain funda- mental programs. We have developed one of these villages as an example of how the regional strategy can be implemented and function in a situation. -
Norway Maps.Pdf
Finnmark lVorwny Trondelag Counties old New Akershus Akershus Bratsberg Telemark Buskerud Buskerud Finnmarken Finnmark Hedemarken Hedmark Jarlsberg Vestfold Kristians Oppland Oppland Lister og Mandal Vest-Agder Nordre Bergenshus Sogn og Fjordane NordreTrondhjem NordTrondelag Nedenes Aust-Agder Nordland Nordland Romsdal Mgre og Romsdal Akershus Sgndre Bergenshus Hordaland SsndreTrondhjem SorTrondelag Oslo Smaalenenes Ostfold Ostfold Stavanger Rogaland Rogaland Tromso Troms Vestfold Aust- Municipal Counties Vest- Agder Agder Kristiania Oslo Bergen Bergen A Feiring ((r Hurdal /\Langset /, \ Alc,ersltus Eidsvoll og Oslo Bjorke \ \\ r- -// Nannestad Heni ,Gi'erdrum Lilliestrom {", {udenes\ ,/\ Aurpkog )Y' ,\ I :' 'lv- '/t:ri \r*r/ t *) I ,I odfltisard l,t Enebakk Nordbv { Frog ) L-[--h il 6- As xrarctaa bak I { ':-\ I Vestby Hvitsten 'ca{a", 'l 4 ,- Holen :\saner Aust-Agder Valle 6rrl-1\ r--- Hylestad l- Austad 7/ Sandes - ,t'r ,'-' aa Gjovdal -.\. '\.-- ! Tovdal ,V-u-/ Vegarshei I *r""i'9^ _t Amli Risor -Ytre ,/ Ssndel Holt vtdestran \ -'ar^/Froland lveland ffi Bergen E- o;l'.t r 'aa*rrra- I t T ]***,,.\ I BYFJORDEN srl ffitt\ --- I 9r Mulen €'r A I t \ t Krohnengen Nordnest Fjellet \ XfC KORSKIRKEN t Nostet "r. I igvono i Leitet I Dokken DOMKIRKEN Dar;sird\ W \ - cyu8npris Lappen LAKSEVAG 'I Uran ,t' \ r-r -,4egry,*T-* \ ilJ]' *.,, Legdene ,rrf\t llruoAs \ o Kirstianborg ,'t? FYLLINGSDALEN {lil};h;h';ltft t)\l/ I t ,a o ff ui Mannasverkl , I t I t /_l-, Fjosanger I ,r-tJ 1r,7" N.fl.nd I r\a ,, , i, I, ,- Buslr,rrud I I N-(f i t\torbo \) l,/ Nes l-t' I J Viker -- l^ -- ---{a - tc')rt"- i Vtre Adal -o-r Uvdal ) Hgnefoss Y':TTS Tryistr-and Sigdal Veggli oJ Rollag ,y Lvnqdal J .--l/Tranbv *\, Frogn6r.tr Flesberg ; \. -

TRANSLATION 1 of 3
114,, Fisheries Pêches TRANSLATION 31 and Oceans et Océans SERIES NO(S) 4888 1 of 3 CANADIAN TRANSLATION OF FISHERIES AND AQUATIC SCIENCES No. 4888 Acid lakes and inland fishing in Norway Results from an interview survey (1974 - 1979) by I.H. Sevaldrud, and I.P. Muniz Original Title: Sure vatn og innlandsfisket i Norge. • Resultater fra intervjuunderseelsene 1974-1979. From: Sur NedbOrs Virkning Pa Skog of Fisk (SNSF-Prosjektet) IR 77/80: 1-203, 1980. Translated by the Translation Bureau (sowF) Multilingual Services Division Department of the Secretary of State of Canada Department of Fisheries and Oceans Northwest Atlantic Fisheries Centre St. John's, NFLD 1982 205 pages typescript Secretary Secrétariat of State d'État MULTILINGUAL SERVICES DIVISION — DIVISION DES SERVICES MULTILINGUES TRANSLATION BUREAU BUREAU DES TRADUCT IONS Iffe LIBRARY IDENTIFICATION — FICHE SIGNALÉTIQUE Translated from - Traduction de Into - En Norwegian English Author - Auteur Iver H. Sevaldrud and Ivar Pors Muniz Title in English or French - Titre anglais ou français Acid Lakes and Inland Fishing in Norway. Results from an Interview Survey (1974 - 1979). Title in foreign language (Transliterate foreign characters) Titre en langue étrangère (Transcrire en caractères romains) Sure vatn og innlandsfisket i Norge. Resultater fra intervjuunders$1(e1sene 1974 - 1979 Reference in foreign language (Name of book or publication) in full, transliterate foreign characters. Référence en langue étrangère (Nom du livre ou publication), au complet, transcrire en caractères romains. Sur nedbç4rs virkning pa skog of fisk (SNSF-prosjektet) Reference in English or French - Référence en anglais ou français • 4eicid Precipitation - Effects on Forest and Fish (the SNSF-project) Publisher - Editeur Page Numbers in original DATE OF PUBLICATION Numéros des pages dans SNSF Project, Box 61, DATE DE PUBLICATION l'original Norway 1432 Aas-NHL, 203 Year Issue No. -
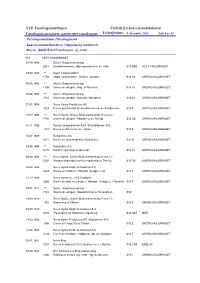
Vassdragene, Stadfest
NVE-Vassdragsavdelingen Utskrift fra konsesjonsdatabasen Vassdragskonsesjoner sortert etter vassdragsnr. Utskriftsdato: 9. desember 2005 Side 1 av 34 Vassdragsområdenr./Vassdragsnavn Konsesjonsdato/Innehaver (Opprinnelig innehaver) Reg.nr. (KDB)/Tittel/Vassdragsnr. og -navn 016 VEST-VASSDRAGET 30.09.1890 Skiens Brugseierforening 2047 Bandaksvannene, slipningsreglement av 1890. 016.BD5 VEST-VASSDRAGET 09.09.1902 ** Skien Cellulosefabrik 1702 Oppd. vannstanden i Åletjern i Gjerpen. 016.A0 SKIENSVASSDRAGET 09.06.1903 ** Skiens Brugseierforening 1708 Skiensvassdraget - Reg. av Møsvatn. 016.J0 SKIENSVASSDRAGET 20.06.1904 ** Skiens Brugseierforening 1710 Skiensvassdraget - Møsvatn (fornyelse) 016.J0 SKIENSVASSDRAGET 27.01.1906 Norsk Hydro Produksjon AS 1829 Erverv og bruksrett på eiendom for utb. av Svelgfossen. 016.F SKIENSVASSDRAGET 18.07.1906 ** Norsk Hydro, Skiens Brukseierforening, Union Co 1716 Skiensvassdraget - Regulering av Tinnsjø 016.G0 SKIENSVASSDRAGET 16.11.1906 Norske Skogindustrier ASA (Klosterfossen A/S) 812 Erverv av Klosterfossen i Skien 016.Z SKIENSVASSDRAGET 10.01.1908 Rjukanfoss A/S 1671 Erverv av vannrettigheter i Rjukanfoss. 016.H SKIENSVASSDRAGET 29.08.1908 ** Rjukanfoss A/S 1672 Utvidet regulering av Møsvatn. 016.J0 SKIENSVASSDRAGET 08.09.1908 ** Norsk Hydro, Skiens Brukseierforening, Union Co 2230 Manøvreringsreglement for regulering av Tinnsjø 016.G0 SKIENSVASSDRAGET 20.08.1909 Norsk Hydro-Elektrisk Kvælstof A/S 1659 Erverv av eiendom i Hitterdal (Svelgfossen). 016.F SKIENSVASSDRAGET 23.12.1909 Norsk Hydro a.s (A/S Svælgfos) 1009 Erverv av deler av Lienfoss i Hiterdal ( Svelgfoss i Tinnelva) 016.F SKIENSVASSDRAGET 20.01.1911 ** Skiens Brugseierforening 1721 Skiensvassdraget - Oppdemming av Hjellevatnet. 016. 19.09.1913 Norsk Hydro, Skiens Brukseierforening, Union Co 893 Regulering av Mårelv 016.Z SKIENSVASSDRAGET 24.09.1915 Norsk Hydro-Elektrisk Kvælstof A/S 1628 Planendring for Mårelvens regulering. -

Homogenization of Norwegian Monthly Precipitation Series for the Period 1961-2018
No. 4/2021 ISSN 2387-4201 METreport Climate Homogenization of Norwegian monthly precipitation series for the period 1961-2018 Elinah Khasandi Kuya, Herdis Motrøen Gjelten, Ole Einar Tveito METreport Title Date Homogenization of Norwegian monthly 2021-04-30 precipitation series for the period 1961-2018 Section Report no. Division for Climate Services No. 4/2021 Author(s) Classification Elinah Khasandi Kuya, Herdis Motrøen Gjelten, ● Free ○ Restricted Ole Einar Tveito Abstract Climatol homogenization method was applied to detect inhomogeneities in Norwegian precipitation series, during the period 1961-2018. 370 series (including 44 from Sweden and one from Finland) of monthly precipitation sums, from the ClimNorm precipitation dataset were used in the analysis. The homogeneity analysis produced a 58-year long homogenous dataset for 325 monthly precipitation sum with regional temporal variability and spatial coherence that is better than that of non-homogenized series. The dataset is more reliable in explaining the large-scale climate variations and was used to calculate the new climate normals in Norway. Keywords Homogenization, climate normals, precipitation Disciplinary signature Responsible signature 2 Abstract Climate normals play an important role in weather and climate studies and therefore require high-quality dataset that is both consistent and homogenous. The Norwegian observation network has changed considerably during the last 20-30 years, introducing non-climatic changes such as automation and relocation. Homogenization was therefore necessary and work has been done to establish a homogeneous precipitation reference dataset for the purpose of calculating the new climatological standard normals for the period 1991-2020. The homogenization tool Climatol was applied to detect inhomogeneities in the Norwegian precipitation series for the period 1961-2018. -

A Four-Phase Model for the Sveconorwegian Orogeny, SW Scandinavia 43
NORWEGIAN JOURNAL OF GEOLOGY A four-phase model for the Sveconorwegian orogeny, SW Scandinavia 43 A four-phase model for the Sveconorwegian orogeny, SW Scandinavia Bernard Bingen, Øystein Nordgulen & Giulio Viola Bingen, B., Nordgulen, Ø. & Viola, G.; A four-phase model for the Sveconorwegian orogeny, SW Scandinavia. Norwegian Journal of Geology vol. 88, pp 43-72. Trondheim 2008. ISSN 029-196X. The Sveconorwegian orogenic belt resulted from collision between Fennoscandia and another major plate, possibly Amazonia, at the end of the Mesoproterozoic. The belt divides, from east to west, into a Paleoproterozoic Eastern Segment, and four mainly Mesoproterozoic terranes trans- ported relative to Fennsocandia. These are the Idefjorden, Kongsberg, Bamble and Telemarkia Terranes. The Eastern Segment is lithologically rela- ted to the Transcandinavian Igneous Belt (TIB), in the Fennoscandian foreland of the belt. The terranes are possibly endemic to Fennoscandia, though an exotic origin for the Telemarkia Terrane is possible. A review of existing geological and geochronological data supports a four-phase Sveconorwegian assembly of these lithotectonic units. (1) At 1140-1080 Ma, the Arendal phase represents the collision between the Idefjorden and Telemarkia Terranes, which produced the Bamble and Kongsberg tectonic wedges. This phase involved closure of an oceanic basin, possibly mar- ginal to Fennoscandia, accretion of a volcanic arc, high-grade metamorphism and deformation in the Bamble and Kongsberg Terranes peaking in granulite-facies conditions at 1140-1125 Ma, and thrusting of the Bamble Terrane onto the Telemarkia Terrane probably at c. 1090-1080 Ma. (2) At 1050-980 Ma, the Agder phase corresponds to the main Sveconorwegian oblique (?) continent-continent collision. -
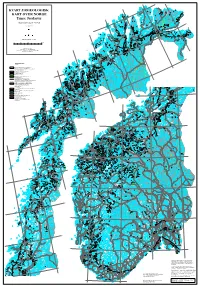
Jordarter V E U N O T N a Leirpollen
30°E 71°N 28°E Austhavet Berlevåg Bearalváhki 26°E Mehamn Nordkinnhalvøya KVARTÆRGEOLOGISK Båtsfjord Vardø D T a e n a Kjøllefjord a n f u j o v r u d o e Oksevatnet t n n KART OVER NORGE a Store L a Buevatnet k Geatnjajávri L s Varangerhalvøya á e Várnjárga f g j e o 24°E Honningsvåg r s d Tema: Jordarter v e u n o t n a Leirpollen Deanodat Vestertana Quaternary map of Norway Havøysund 70°N en rd 3. opplag 2013 fjo r D e a T g tn e n o a ra u a a v n V at t j j a n r á u V Porsanger- Vadsø Vestre Kjæsvatnet Jakobselv halvøya o n Keaisajávri Geassájávri o Store 71°N u Bordejávrrit v Måsvatn n n i e g Havvannet d n r evsbotn R a o j s f r r Kjø- o Bugøy- e fjorden g P fjorden 22°E n a Garsjøen Suolo- s r Kirkenes jávri o Mohkkejávri P Sandøy- Hammerfest Hesseng fjorden Rypefjord t Bjørnevatn e d n Målestokk (Scale) 1:1 mill. u Repparfjorden s y ø r ø S 0 25 50 100 Km Sørøya Sør-Varanger Sállan Skáiddejávri Store Porsanger Sametti Hasvik Leaktojávri Kartet inngår også i B áhèeveai- NASJONALATLAS FOR NORGE 20°E Leavdnja johka u a Lopphavet -

Miljødirektoratets Tilråding Sommer 2017.Pdf
MILJØDIREKTORATET SIN TILRÅDING TIL KLIMA- OG MILJØDEPARTEMENTET OM VERNEPLAN FOR SKOG SOMMER 2017 August 2017 1 1 FORSLAG Miljødirektoratet tilrår vern av 36 naturreservater i skog i medhold av naturmangfoldloven (lov om forvaltning av naturens mangfold). 26 av områdene er nye naturreservater, 10 av områdene er utvidelse av eksisterende naturreservater. For 3 av områdene foreslås utvidelsen som endring av eksisterende verneforskrift. Ett av områdene ligger på Statskog SF sin grunn i Sør-Trøndelag fylke, mens de øvrige områdene inngår i ordningen med frivillig vern av privateid skog. Tilrådingen omfatter ca. 75,3 km2 nytt verneareal, hvorav ca 60 km2 er produktiv skog. Områdene som foreslås vernet er: 1. Linddalsfjellet og Sydalen i Evje og Hornnes kommune, Aust-Agder fylke 2. Torehei (utvidelse av Røyrmyråsen naturreservat) i Lillesand kommune, Aust-Agder fylke 3. Styggetjønnåsen i Froland kommune, Aust-Agder fylke 4. Haresteinheia i Froland kommune, Aust-Agder fylke 5. Romeheia i Froland kommune, Aust-Agder fylke 6. Bjoruvstøl i Bygland kommune, Aust-Agder fylke 7. Kvåsfossen i Lyngdal kommune, Vest-Agder fylke 8. Utvidelse av Lone naturreservat i Drangedal kommune, Telemark fylke 9. Utvidelse av Asgjerdstigfjell naturreservat i Drangedal kommune, Telemark fylke 10. Malfjell i Drangedal kommune, Telemark fylke 11. Sandvikheia i Drangedal kommune, Telemark fylke 12. Vedfallnosa i Drangedal kommune, Telemark fylke 13. Holtsåsen i Porsgrunn kommune, Telemark fylke 14. Høgvollane i Skien kommune, Telemark fylke 15. Fisketjønnjuvet i Kviteseid kommune, Telemark fylke 16. Nedre Rønningkåsene i Nome kommune, Telemark fylke 17. Nordre Lia i Fyresdal kommune, Telemark fylke 18. Utvidelse av Solhomfjell og Kvenntjønnane naturreservat i Nissedal og Gjerstad kommuner, Telemark og Aust-Agder fylker 19.