Translesion DNA Polymerases Remodel the Replisome and Alter the Speed of the Replicative Helicase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Replisome Assembly at Oric, the Replication Origin of E. Coli, Reveals an Explanation for Initiation Sites Outside an Origin
Molecular Cell, Vol. 4, 541±553, October, 1999, Copyright 1999 by Cell Press Replisome Assembly at oriC, the Replication Origin of E. coli, Reveals an Explanation for Initiation Sites outside an Origin Linhua Fang,*§ Megan J. Davey,² and Mike O'Donnell²³ have not been addressed. For example, is the local un- *Microbiology Department winding sufficiently large for two helicases to assemble Joan and Sanford I. Weill Graduate School of Medical for bidirectional replication, or does one helicase need Sciences of Cornell University to enter first and expand the bubble via helicase action New York, New York 10021 to make room for the second helicase? The known rep- ² The Rockefeller University and licative helicases are hexameric and encircle ssDNA. Howard Hughes Medical Institute Which strand does the initial helicase(s) at the origin New York, New York 10021 encircle, and if there are two, how are they positioned relative to one another? Primases generally require at least transient interaction with helicase to function. Can Summary primase function with the helicase(s) directly after heli- case assembly at the origin, or must helicase-catalyzed This study outlines the events downstream of origin DNA unwinding occur prior to RNA primer synthesis? unwinding by DnaA, leading to assembly of two repli- Chromosomal replicases are comprised of a ring-shaped cation forks at the E. coli origin, oriC. We show that protein clamp that encircles DNA, a clamp-loading com- two hexamers of DnaB assemble onto the opposing plex that uses ATP to assemble the clamp around DNA, strands of the resulting bubble, expanding it further, and a DNA polymerase that binds the circular clamp, yet helicase action is not required. -
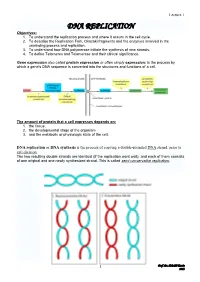
DNA REPLICATION Objectives: 1
Lecture 1 DNA REPLICATION Objectives: 1. To understand the replication process and where it occurs in the cell cycle. 2. To describe the Replication Fork, Okazaki fragments and the enzymes involved in the unwinding process and replication. 3. To understand how DNA polymerase initiate the synthesis of new strands. 4. To define Telomeres and Telomerase and their clinical significance. Gene expression also called protein expression or often simply expression: is the process by which a gene's DNA sequence is converted into the structures and functions of a cell. The amount of protein that a cell expresses depends on: 1. the tissue, 2. the developmental stage of the organism 3. and the metabolic or physiologic state of the cell. DNA replication or DNA synthesis is the process of copying a double-stranded DNA strand, prior to cell division. The two resulting double strands are identical (if the replication went well), and each of them consists of one original and one newly synthesized strand. This is called semi conservative replication. 1 Prof. Dr. H.D.El-Yassin 2013 Lecture 1 The process of replication consists of three steps, initiation, replication and termination. 1. Prokaryotic replication Basic Requirement for DNA Synthesis 1. Substrates: the four deoxy nucleosides triphosphates are needed as substrates for DNA synthesis. Cleavage of the high-energy phosphate bond between the α and β phosphates provides the energy for the addition of the nucleotide. 2. Template: DNA replication cannot occur without a template. A template is required to direct the addition of the appropriate complementary deoxynucleotide to the newly synthesized DNA strand. -
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kath, James Evon. 2016. DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26718716 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! ! ! ! ! ! ! DNA!polymerase!exchange!and!lesion!bypass!in!Escherichia)coli! ! A!dissertation!presented! by! James!Evon!Kath! to! The!Committee!on!Higher!Degrees!in!Biophysics! ! in!partial!fulfillment!of!the!requirements! for!the!degree!of! Doctor!of!Philosophy! in!the!subject!of! Biophysics! ! Harvard!University! Cambridge,!Massachusetts! October!2015! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ©!2015!L!James!E.!Kath.!Some!Rights!Reserved.! ! This!work!is!licensed!under!the!Creative!Commons!Attribution!3.0!United!States!License.!To! view!a!copy!of!this!license,!visit:!http://creativecommons.org/licenses/By/3.0/us! ! ! Dissertation!Advisor:!Professor!Joseph!J.!Loparo! ! ! !!!!!!!!James!Evon!Kath! ! DNA$polymerase$exchange$and$lesion$bypass$in$Escherichia)coli$ $ Abstract$ ! Translesion! synthesis! (TLS)! alleviates! -

USP7 Couples DNA Replication Termination to Mitotic Entry
bioRxiv preprint doi: https://doi.org/10.1101/305318; this version posted April 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. USP7 couples DNA replication termination to mitotic entry Antonio Galarreta1*, Emilio Lecona1*, Pablo Valledor1, Patricia Ubieto1,2, Vanesa Lafarga1, Julia Specks1 & Oscar Fernandez-Capetillo1,3 1Genomic Instability Group, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain 2Current Address: DNA Replication Group, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain 3Science for Life Laboratory, Division of Genome Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institute, S-171 21 Stockholm, Sweden *Co-first authors Correspondence: E.L. ([email protected]) or O.F. ([email protected]) Lead Contact: Oscar Fernandez-Capetillo Spanish National Cancer Research Centre (CNIO) Melchor Fernandez Almagro, 3 Madrid 28029, Spain Tel.: +34.91.732.8000 Ext: 3480 Fax: +34.91.732.8028 Email: [email protected] KEYWORDS: USP7; CDK1; DNA REPLICATION; MITOSIS; S/M TRANSITION. bioRxiv preprint doi: https://doi.org/10.1101/305318; this version posted April 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. USP7 coordinates the S/M transition 2 SUMMARY To ensure a faithful segregation of chromosomes, DNA must be fully replicated before mitotic entry. However, how cells sense the completion of DNA replication and to what extent this is linked to the activation of the mitotic machinery remains poorly understood. We previously showed that USP7 is a replisome-associated deubiquitinase with an essential role in DNA replication. -

Phosphatidylinositol-3-Kinase Related Kinases (Pikks) in Radiation-Induced Dna Damage
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2012, vol. 81(4), p. 177-187 ISSN 0372-7025 DOI: 10.31482/mmsl.2012.025 REVIEW ARTICLE PHOSPHATIDYLINOSITOL-3-KINASE RELATED KINASES (PIKKS) IN RADIATION-INDUCED DNA DAMAGE Ales Tichy 1, Kamila Durisova 1, Eva Novotna 1, Lenka Zarybnicka 1, Jirina Vavrova 1, Jaroslav Pejchal 2, Zuzana Sinkorova 1 1 Department of Radiobiology, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic 2 Centrum of Advanced Studies, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic. Received 5 th September 2012. Revised 27 th November 2012. Published 7 th December 2012. Summary This review describes a drug target for cancer therapy, family of phosphatidylinositol-3 kinase related kinases (PIKKs), and it gives a comprehensive review of recent information. Besides general information about phosphatidylinositol-3 kinase superfamily, it characterizes a DNA-damage response pathway since it is monitored by PIKKs. Key words: PIKKs; ATM; ATR; DNA-PK; Ionising radiation; DNA-repair ABBREVIATIONS therapy and radiation play a pivotal role. Since cancer is one of the leading causes of death worldwide, it is DSB - double stand breaks, reasonable to invest time and resources in the enligh - IR - ionising radiation, tening of mechanisms, which underlie radio-resis - p53 - TP53 tumour suppressors, tance. PI - phosphatidylinositol. The aim of this review is to describe the family INTRODUCTION of phosphatidyinositol 3-kinases (PI3K) and its func - tional subgroup - phosphatidylinositol-3-kinase rela - An efficient cancer treatment means to restore ted kinases (PIKKs) and their relation to repairing of controlled tissue growth via interfering with cell sig - radiation-induced DNA damage. -

Collision with Duplex DNA Renders Escherichia Coli DNA Polymerase III
www.nature.com/scientificreports OPEN Collision with duplex DNA renders Escherichia coli DNA polymerase III holoenzyme susceptible to Received: 8 May 2017 Accepted: 18 September 2017 DNA polymerase IV-mediated Published: xx xx xxxx polymerase switching on the sliding clamp Thanh Thi Le, Asako Furukohri, Masahiro Tatsumi-Akiyama & Hisaji Maki Organisms possess multiple DNA polymerases (Pols) and use each for a different purpose. One of the five Pols inEscherichia coli, DNA polymerase IV (Pol IV), encoded by the dinB gene, is known to participate in lesion bypass at certain DNA adducts. To understand how cells choose Pols when the replication fork encounters an obstacle on template DNA, the process of polymerase exchange from the primary replicative enzyme DNA polymerase III (Pol III) to Pol IV was studied in vitro. Replicating Pol III forming a tight holoenzyme (Pol III HE) with the sliding clamp was challenged by Pol IV on a primed ssDNA template carrying a short inverted repeat. A rapid and lesion-independent switch from Pol III to Pol IV occurred when Pol III HE encountered a hairpin stem duplex, implying that the loss of Pol III-ssDNA contact induces switching to Pol IV. Supporting this idea, mutant Pol III with an increased affinity for ssDNA was more resistant to Pol IV than wild-type Pol III was. We observed that an exchange between Pol III and Pol IV also occurred when Pol III HE collided with primer/template duplex. Our data suggest that Pol III-ssDNA interaction may modulate the susceptibility of Pol III HE to Pol IV-mediated polymerase exchange. -

Polymerase Δ Deficiency Causes Syndromic Immunodeficiency with Replicative Stress
Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde, … , Mirjam van der Burg, Kaan Boztug J Clin Invest. 2019. https://doi.org/10.1172/JCI128903. Research Article Genetics Immunology Graphical abstract Find the latest version: https://jci.me/128903/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde,1,2 Özlem Yüce Petronczki,1,2,3 Safa Baris,4,5 Katharina L. Willmann,1,2 Enrico Girardi,2 Elisabeth Salzer,1,2,3,6 Stefan Weitzer,7 Rico Chandra Ardy,1,2,3 Ana Krolo,1,2,3 Hanna Ijspeert,8 Ayca Kiykim,4,5 Elif Karakoc-Aydiner,4,5 Elisabeth Förster-Waldl,9 Leo Kager,6 Winfried F. Pickl,10 Giulio Superti-Furga,2,11 Javier Martínez,7 Joanna I. Loizou,2 Ahmet Ozen,4,5 Mirjam van der Burg,8 and Kaan Boztug1,2,3,6 1Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, 2CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, and 3St. Anna Children’s Cancer Research Institute (CCRI), Vienna, Austria. 4Pediatric Allergy and Immunology, Marmara University, Faculty of Medicine, Istanbul, Turkey. 5Jeffrey Modell Diagnostic Center for Primary Immunodeficiency Diseases, Marmara University, Istanbul, Turkey. 6St. Anna Children’s Hospital, Department of Pediatrics and Adolescent Medicine, Vienna, Austria. 7Center for Medical Biochemistry, Medical University of Vienna, Vienna, Austria. 8Department of Pediatrics, Laboratory for Immunology, Leiden University Medical Centre, Leiden, Netherlands. 9Department of Neonatology, Pediatric Intensive Care and Neuropediatrics, Department of Pediatrics and Adolescent Medicine, 10Institute of Immunology, Center for Pathophysiology, Infectiology and Immunology, and 11Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria. -

The Obscure World of Integrative and Mobilizable Elements Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget
The obscure world of integrative and mobilizable elements Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget To cite this version: Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget. The obscure world of integrative and mobilizable elements: Highly widespread elements that pirate bacterial conjugative systems. Genes, MDPI, 2017, 8 (11), pp.337. 10.3390/genes8110337. hal- 01686871 HAL Id: hal-01686871 https://hal.archives-ouvertes.fr/hal-01686871 Submitted on 26 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License G C A T T A C G G C A T genes Review The Obscure World of Integrative and Mobilizable Elements, Highly Widespread Elements that Pirate Bacterial Conjugative Systems Gérard Guédon *, Virginie Libante, Charles Coluzzi, Sophie Payot and Nathalie Leblond-Bourget * ID DynAMic, Université de Lorraine, INRA, 54506 Vandœuvre-lès-Nancy, France; [email protected] (V.L.); [email protected] (C.C.); [email protected] (S.P.) * Correspondence: [email protected] (G.G.); [email protected] (N.L.-B.); Tel.: +33-037-274-5142 (G.G.); +33-037-274-5146 (N.L.-B.) Received: 12 October 2017; Accepted: 15 November 2017; Published: 22 November 2017 Abstract: Conjugation is a key mechanism of bacterial evolution that involves mobile genetic elements. -

Conversion of OX174 and Fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia Coli (Dnac, Dnad, and Dnag Gene Products/DNA Polymerase III) REED B
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3233-3237, November 1972 Conversion of OX174 and fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia coli (dnaC, dnaD, and dnaG gene products/DNA polymerase III) REED B. WICKNER, MICHEL WRIGHT, SUE WICKNER, AND JERARD HURWITZ Department of Developmental Biology and Cancer, Division of Biological Sciences, Albert Einstein College of Medicine, Bronx, New York 10461 Communicated by Harry Eagle, August 28, 1972 ABSTRACT 4X174 and M13 (fd) single-stranded cir- MATERIALS AND METHODS cular DNAs are converted to their replicative forms by ex- tracts of E. coli pol Al cells. We find that the qX174 DNA- [a-32P]dTTP was obtained from New England Nuclear Corp. dependent reaction requires Mg++, ATP, and all four de- OX174 DNA was prepared by the method of Sinsheimer (7) oxynucleoside triphosphates, but not CTP, UTP, or GTP. or Franke and Ray (8), while fd viral DNA was prepared as This reaction also involves the products of the dnaC, dnaD, dnaE (DNA polymerase III), and dnaG genes, described (9). Pancreatic RNase was the highest grade ob- but not that of dnaF (ribonucleotide reductase). The in tainable from Worthington Biochemical Corp. It was further vitro conversion of fd single-stranded DNA to the replica- freed of possible contaminating DNase by heating a solution tive form requires all four ribonucleoside triphosphates, (2 mg/ml in 15 mM sodium citrate, pH 5) at 800 for 10 min. Mg++, and all four deoxynucleoside triphosphates. The E. protein was purified from E. coli strain reaction involves the product of gene dnaE but not those coli unwinding of genes dnaC, dnaD, dnaF, or dnaG. -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Product Sheet Info
Master Clone List for NR-19279 ® Vibrio cholerae Gateway Clone Set, Recombinant in Escherichia coli, Plates 1-46 Catalog No. NR-19279 Table 1: Vibrio cholerae Gateway® Clones, Plate 1 (NR-19679) Clone ID Well ORF Locus ID Symbol Product Accession Position Length Number 174071 A02 367 VC2271 ribD riboflavin-specific deaminase NP_231902.1 174346 A03 336 VC1877 lpxK tetraacyldisaccharide 4`-kinase NP_231511.1 174354 A04 342 VC0953 holA DNA polymerase III, delta subunit NP_230600.1 174115 A05 388 VC2085 sucC succinyl-CoA synthase, beta subunit NP_231717.1 174310 A06 506 VC2400 murC UDP-N-acetylmuramate--alanine ligase NP_232030.1 174523 A07 132 VC0644 rbfA ribosome-binding factor A NP_230293.2 174632 A08 322 VC0681 ribF riboflavin kinase-FMN adenylyltransferase NP_230330.1 174930 A09 433 VC0720 phoR histidine protein kinase PhoR NP_230369.1 174953 A10 206 VC1178 conserved hypothetical protein NP_230823.1 174976 A11 213 VC2358 hypothetical protein NP_231988.1 174898 A12 369 VC0154 trmA tRNA (uracil-5-)-methyltransferase NP_229811.1 174059 B01 73 VC2098 hypothetical protein NP_231730.1 174075 B02 82 VC0561 rpsP ribosomal protein S16 NP_230212.1 174087 B03 378 VC1843 cydB-1 cytochrome d ubiquinol oxidase, subunit II NP_231477.1 174099 B04 383 VC1798 eha eha protein NP_231433.1 174294 B05 494 VC0763 GTP-binding protein NP_230412.1 174311 B06 314 VC2183 prsA ribose-phosphate pyrophosphokinase NP_231814.1 174603 B07 108 VC0675 thyA thymidylate synthase NP_230324.1 174474 B08 466 VC1297 asnS asparaginyl-tRNA synthetase NP_230942.2 174933 B09 198 -

Non-Canonical Functions of the Bacterial Sos Response
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2019 Non-Canonical Functions Of The aB cterial Sos Response Amanda Samuels University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Microbiology Commons Recommended Citation Samuels, Amanda, "Non-Canonical Functions Of The aB cterial Sos Response" (2019). Publicly Accessible Penn Dissertations. 3253. https://repository.upenn.edu/edissertations/3253 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/3253 For more information, please contact [email protected]. Non-Canonical Functions Of The aB cterial Sos Response Abstract DNA damage is a pervasive environmental threat, as such, most bacteria encode a network of genes called the SOS response that is poised to combat genotoxic stress. In the absence of DNA damage, the SOS response is repressed by LexA, a repressor-protease. In the presence of DNA damage, LexA undergoes a self-cleavage reaction relieving repression of SOS-controlled effector genes that promote bacterial survival. However, depending on the bacterial species, the SOS response has an expanded role beyond DNA repair, regulating genes involved in mutagenesis, virulence, persistence, and inter-species competition. Despite a plethora of research describing the significant consequences of the SOS response, it remains unknown what physiologic environments induce and require the SOS response for bacterial survival. In Chapter 2, we utilize a commensal E. coli strain, MP1, and established that the SOS response is critical for sustained colonization of the murine gut. Significantly, in evaluating the origin of the genotoxic stress, we found that the SOS response was nonessential for successful colonization in the absence of the endogenous gut microbiome, suggesting that competing microbes might be the source of genotoxic stress.