Roles of DNA Polymerases V and II in SOS-Induced Error-Prone and Error-Free Repair in Escherichia Coli
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
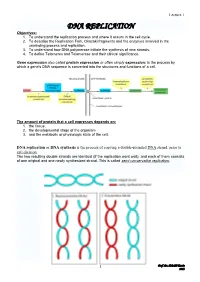
DNA REPLICATION Objectives: 1
Lecture 1 DNA REPLICATION Objectives: 1. To understand the replication process and where it occurs in the cell cycle. 2. To describe the Replication Fork, Okazaki fragments and the enzymes involved in the unwinding process and replication. 3. To understand how DNA polymerase initiate the synthesis of new strands. 4. To define Telomeres and Telomerase and their clinical significance. Gene expression also called protein expression or often simply expression: is the process by which a gene's DNA sequence is converted into the structures and functions of a cell. The amount of protein that a cell expresses depends on: 1. the tissue, 2. the developmental stage of the organism 3. and the metabolic or physiologic state of the cell. DNA replication or DNA synthesis is the process of copying a double-stranded DNA strand, prior to cell division. The two resulting double strands are identical (if the replication went well), and each of them consists of one original and one newly synthesized strand. This is called semi conservative replication. 1 Prof. Dr. H.D.El-Yassin 2013 Lecture 1 The process of replication consists of three steps, initiation, replication and termination. 1. Prokaryotic replication Basic Requirement for DNA Synthesis 1. Substrates: the four deoxy nucleosides triphosphates are needed as substrates for DNA synthesis. Cleavage of the high-energy phosphate bond between the α and β phosphates provides the energy for the addition of the nucleotide. 2. Template: DNA replication cannot occur without a template. A template is required to direct the addition of the appropriate complementary deoxynucleotide to the newly synthesized DNA strand. -
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kath, James Evon. 2016. DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26718716 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! ! ! ! ! ! ! DNA!polymerase!exchange!and!lesion!bypass!in!Escherichia)coli! ! A!dissertation!presented! by! James!Evon!Kath! to! The!Committee!on!Higher!Degrees!in!Biophysics! ! in!partial!fulfillment!of!the!requirements! for!the!degree!of! Doctor!of!Philosophy! in!the!subject!of! Biophysics! ! Harvard!University! Cambridge,!Massachusetts! October!2015! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ©!2015!L!James!E.!Kath.!Some!Rights!Reserved.! ! This!work!is!licensed!under!the!Creative!Commons!Attribution!3.0!United!States!License.!To! view!a!copy!of!this!license,!visit:!http://creativecommons.org/licenses/By/3.0/us! ! ! Dissertation!Advisor:!Professor!Joseph!J.!Loparo! ! ! !!!!!!!!James!Evon!Kath! ! DNA$polymerase$exchange$and$lesion$bypass$in$Escherichia)coli$ $ Abstract$ ! Translesion! synthesis! (TLS)! alleviates! -

DNA Polymerase V Activity Is Autoregulated by a Novel Intrinsic DNA-Dependent
1 2 DNA polymerase V activity is autoregulated by a novel intrinsic DNA-dependent 3 ATPase 4 Aysen L. Erdem1, Malgorzata Jaszczur1, Jeffrey G. Bertram1, Roger Woodgate2, Michael M. Cox3 & 5 Myron F. Goodman1 6 1Departments of Biological Sciences and Chemistry, University of Southern California, University 7 Park, Los Angeles, California 90089-2910, USA. 2Laboratory of Genomic Integrity, National 8 Institute of Child Health and Human Development, National Institutes of Health, Bethesda, 9 Maryland 20892-3371, USA. 3Department of Biochemistry, University of Wisconsin-Madison, 10 Madison, Wisconsin 53706, USA. 11 12 Escherichia coli DNA polymerase V (pol V), a heterotrimeric complex composed of UmuD′2C, 13 is marginally active. ATP and RecA play essential roles in the activation of pol V for DNA 14 synthesis including translesion synthesis (TLS). We have established three features of the roles 15 of ATP and RecA. 1) RecA-activated DNA polymerase V (pol V Mut), is a DNA-dependent 16 ATPase; 2) bound ATP is required for DNA synthesis; 3) pol V Mut function is regulated by 17 ATP, with ATP required to bind primer/template (p/t) DNA and ATP hydrolysis triggering 18 dissociation from the DNA. Pol V Mut formed with an ATPase-deficient RecA E38K/K72R 19 mutant hydrolyzes ATP rapidly, establishing the DNA-dependent ATPase as an intrinsic 20 property of pol V Mut distinct from the ATP hydrolytic activity of RecA when bound to 21 single-stranded (ss)DNA as a nucleoprotein filament (RecA*). No similar ATPase activity or 22 autoregulatory mechanism has previously been found for a DNA polymerase. -

Reca Acts As a Switch to Regulate Polymerase Occupancy in a Moving Replication Fork
RecA acts as a switch to regulate polymerase occupancy in a moving replication fork Chiara Indiania,1, Meghna Patelb, Myron F. Goodmanb, and Mike E. O’Donnellc,1 aManhattan College, Riverdale, NY 10471; bDepartment of Biological Sciences, University of Southern California, Los Angeles, CA 90089; and cHoward Hughes Medical Institute, Rockefeller University, New York, NY 10065 Contributed by Mike O’Donnell, February 19, 2013 (sent for review February 6, 2013) This report discovers a role of Escherichia coli RecA, the cellular regulates DNA polymerase access to the replication fork. Sur- recombinase, in directing the action of several DNA polymerases prisingly, RecA strongly inhibits fork progression by Pol III repli- at the replication fork. Bulk chromosome replication is performed somes, yet RecA stimulates TLS Pol II and Pol IV replisomes. The by DNA polymerase (Pol) III. However, E. coli contains translesion mechanism that underlies the opposite effects of RecA on Pol III synthesis (TLS) Pols II, IV, and V that also function with the helicase, and the TLS Pol replisomes involves single-strand binding protein primase, and sliding clamp in the replisome. Surprisingly, we find (SSB). Although all of the polymerases function with SSB on the that RecA specifically activates replisomes that contain TLS Pols. In template strand, SSB inhibits TLS Pol function in the context of sharp contrast, RecA severely inhibits the Pol III replisome. Given a replisome, presumably acting in trans by binding lagging-strand the opposite effects of RecA on Pol III and TLS replisomes, we pro- ssDNA. RecA relieves the SSB induced repression of TLS Pol pose that RecA acts as a switch to regulate the occupancy of poly- replisomes and stimulates their action, while inhibiting the Pol III merases within a moving replisome. -

Y-Family DNA Polymerases in Escherichia Coli
Y-family DNA polymerases in Escherichia coli The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Jarosz, Daniel F. et al. “Y-family DNA Polymerases in Escherichia Coli.” Trends in Microbiology 15.2 (2007): 70–77. Web. 13 Apr. 2012. © 2007 Elsevier Ltd. As Published http://dx.doi.org/10.1016/j.tim.2006.12.004 Publisher Elsevier Version Final published version Citable link http://hdl.handle.net/1721.1/70041 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Review TRENDS in Microbiology Vol.15 No.2 Y-family DNA polymerases in Escherichia coli Daniel F. Jarosz1, Penny J. Beuning2,3, Susan E. Cohen2 and Graham C. Walker2 1 Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 2 Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 3 Department of Chemistry and Chemical Biology, Northeastern University, Boston, MA 02115, USA The observation that mutations in the Escherichia coli that is also lexA-independent [4]. This might have genes umuC+ and umuD+ abolish mutagenesis induced particularly important implications for bacteria living by UV light strongly supported the counterintuitive under conditions of nutrient starvation. The SOS response notion that such mutagenesis is an active rather than also seems to be oscillatory at the single-cell level, and this passive process. Genetic and biochemical studies have oscillation is dependent on the umuDC genes [5]. -

DNA Polymerase Zeta-Dependent Mutagenesis: Molecular Specificity, Extent of Error-Prone Synthesis, and the Role of Dntp Pools
University of Nebraska Medical Center DigitalCommons@UNMC Theses & Dissertations Graduate Studies Fall 12-16-2016 DNA Polymerase Zeta-Dependent Mutagenesis: Molecular Specificity, Extent of Error-Prone Synthesis, and the Role of dNTP Pools Olga V. Kochenova University of Nebraska Medical Center Follow this and additional works at: https://digitalcommons.unmc.edu/etd Part of the Biochemistry Commons, Genetics Commons, Molecular Biology Commons, and the Molecular Genetics Commons Recommended Citation Kochenova, Olga V., "DNA Polymerase Zeta-Dependent Mutagenesis: Molecular Specificity, Extent of Error- Prone Synthesis, and the Role of dNTP Pools" (2016). Theses & Dissertations. 153. https://digitalcommons.unmc.edu/etd/153 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@UNMC. It has been accepted for inclusion in Theses & Dissertations by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. DNA POLYMERASE ζ -DEPENDENT MUTAGENESIS: MOLECULAR SPECIFICITY, EXTENT OF ERROR-PRONE SYNTHESIS, AND THE ROLE OF dNTP POOLS by Olga Kochenova A DISSERTATION Presented to the Faculty of The University of Nebraska Graduate College In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy Cancer Research Graduate Program Under the Supervision of Professor Polina V. Shcherbakova University of Nebraska Medical Center Omaha, Nebraska October, 2016 Supervisory Committee: Youri Pavlov, Ph. D. Tadayoshi Bessho, Ph. D. Michel Ouellette, Ph. D. DNA POLYMERASE ζ -DEPENDENT MUTAGENESIS: MOLECULAR SPECIFICITY, EXTENT OF ERROR-PRONE SYNTHESIS, AND THE ROLE OF dNTP POOLS Olga V. Kochenova, Ph.D. University of Nebraska, 2016 Supervisor: Polina V. Shcherbakova, Ph.D. Despite multiple DNA repair pathways, DNA lesions can escape repair and compromise normal chromosomal replication, leading to genome instability. -

Conversion of OX174 and Fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia Coli (Dnac, Dnad, and Dnag Gene Products/DNA Polymerase III) REED B
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3233-3237, November 1972 Conversion of OX174 and fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia coli (dnaC, dnaD, and dnaG gene products/DNA polymerase III) REED B. WICKNER, MICHEL WRIGHT, SUE WICKNER, AND JERARD HURWITZ Department of Developmental Biology and Cancer, Division of Biological Sciences, Albert Einstein College of Medicine, Bronx, New York 10461 Communicated by Harry Eagle, August 28, 1972 ABSTRACT 4X174 and M13 (fd) single-stranded cir- MATERIALS AND METHODS cular DNAs are converted to their replicative forms by ex- tracts of E. coli pol Al cells. We find that the qX174 DNA- [a-32P]dTTP was obtained from New England Nuclear Corp. dependent reaction requires Mg++, ATP, and all four de- OX174 DNA was prepared by the method of Sinsheimer (7) oxynucleoside triphosphates, but not CTP, UTP, or GTP. or Franke and Ray (8), while fd viral DNA was prepared as This reaction also involves the products of the dnaC, dnaD, dnaE (DNA polymerase III), and dnaG genes, described (9). Pancreatic RNase was the highest grade ob- but not that of dnaF (ribonucleotide reductase). The in tainable from Worthington Biochemical Corp. It was further vitro conversion of fd single-stranded DNA to the replica- freed of possible contaminating DNase by heating a solution tive form requires all four ribonucleoside triphosphates, (2 mg/ml in 15 mM sodium citrate, pH 5) at 800 for 10 min. Mg++, and all four deoxynucleoside triphosphates. The E. protein was purified from E. coli strain reaction involves the product of gene dnaE but not those coli unwinding of genes dnaC, dnaD, dnaF, or dnaG. -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Endogenous Overexpression of an Active Phosphorylated Form of DNA Polymerase Β Under Oxidative Stress in Trypanosoma Cruzi
RESEARCH ARTICLE Endogenous overexpression of an active phosphorylated form of DNA polymerase β under oxidative stress in Trypanosoma cruzi Diego A. Rojas1☯, Fabiola Urbina2☯, Sandra Moreira-Ramos2, Christian Castillo3, Ulrike Kemmerling3, Michel Lapier4, Juan Diego Maya4, Aldo Solari2, Edio Maldonado2¤* 1 Microbiology and Micology Program, ICBM, Faculty of Medicine, University of Chile, Santiago, Chile, 2 Cellular and Molecular Biology Program, ICBM, Faculty of Medicine, University of Chile, Santiago, Chile, 3 Anatomy and Developmental Biology Program, ICBM, Faculty of Medicine, University of Chile, Santiago, a1111111111 Chile, 4 Molecular and Clinical Pharmacology Program, ICBM, Faculty of Medicine, University of Chile, a1111111111 Santiago, Chile a1111111111 a1111111111 ☯ These authors contributed equally to this work. a1111111111 ¤ Current address: Independencia, Santiago, Chile * [email protected] Abstract OPEN ACCESS Trypanosoma cruzi is exposed during its life to exogenous and endogenous oxidative Citation: Rojas DA, Urbina F, Moreira-Ramos S, Castillo C, Kemmerling U, Lapier M, et al. (2018) stress, leading to damage of several macromolecules such as DNA. There are many DNA Endogenous overexpression of an active repair pathways in the nucleus and mitochondria (kinetoplast), where specific protein com- phosphorylated form of DNA polymerase β under plexes detect and eliminate damage to DNA. One group of these proteins is the DNA poly- oxidative stress in Trypanosoma cruzi. PLoS Negl Trop Dis 12(2): e0006220. https://doi.org/10.1371/ merases. In particular, Tc DNA polymerase β participates in kinetoplast DNA replication and journal.pntd.0006220 repair. However, the mechanisms which control its expression under oxidative stress are Editor: Joachim Clos, Bernhard Nocht Institute for still unknown. -

Product Sheet Info
Master Clone List for NR-19279 ® Vibrio cholerae Gateway Clone Set, Recombinant in Escherichia coli, Plates 1-46 Catalog No. NR-19279 Table 1: Vibrio cholerae Gateway® Clones, Plate 1 (NR-19679) Clone ID Well ORF Locus ID Symbol Product Accession Position Length Number 174071 A02 367 VC2271 ribD riboflavin-specific deaminase NP_231902.1 174346 A03 336 VC1877 lpxK tetraacyldisaccharide 4`-kinase NP_231511.1 174354 A04 342 VC0953 holA DNA polymerase III, delta subunit NP_230600.1 174115 A05 388 VC2085 sucC succinyl-CoA synthase, beta subunit NP_231717.1 174310 A06 506 VC2400 murC UDP-N-acetylmuramate--alanine ligase NP_232030.1 174523 A07 132 VC0644 rbfA ribosome-binding factor A NP_230293.2 174632 A08 322 VC0681 ribF riboflavin kinase-FMN adenylyltransferase NP_230330.1 174930 A09 433 VC0720 phoR histidine protein kinase PhoR NP_230369.1 174953 A10 206 VC1178 conserved hypothetical protein NP_230823.1 174976 A11 213 VC2358 hypothetical protein NP_231988.1 174898 A12 369 VC0154 trmA tRNA (uracil-5-)-methyltransferase NP_229811.1 174059 B01 73 VC2098 hypothetical protein NP_231730.1 174075 B02 82 VC0561 rpsP ribosomal protein S16 NP_230212.1 174087 B03 378 VC1843 cydB-1 cytochrome d ubiquinol oxidase, subunit II NP_231477.1 174099 B04 383 VC1798 eha eha protein NP_231433.1 174294 B05 494 VC0763 GTP-binding protein NP_230412.1 174311 B06 314 VC2183 prsA ribose-phosphate pyrophosphokinase NP_231814.1 174603 B07 108 VC0675 thyA thymidylate synthase NP_230324.1 174474 B08 466 VC1297 asnS asparaginyl-tRNA synthetase NP_230942.2 174933 B09 198 -

Supplementary Information
Supplementary information (a) (b) Figure S1. Resistant (a) and sensitive (b) gene scores plotted against subsystems involved in cell regulation. The small circles represent the individual hits and the large circles represent the mean of each subsystem. Each individual score signifies the mean of 12 trials – three biological and four technical. The p-value was calculated as a two-tailed t-test and significance was determined using the Benjamini-Hochberg procedure; false discovery rate was selected to be 0.1. Plots constructed using Pathway Tools, Omics Dashboard. Figure S2. Connectivity map displaying the predicted functional associations between the silver-resistant gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S3. Connectivity map displaying the predicted functional associations between the silver-sensitive gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S4. Metabolic overview of the pathways in Escherichia coli. The pathways involved in silver-resistance are coloured according to respective normalized score. Each individual score represents the mean of 12 trials – three biological and four technical. Amino acid – upward pointing triangle, carbohydrate – square, proteins – diamond, purines – vertical ellipse, cofactor – downward pointing triangle, tRNA – tee, and other – circle. -

Development of Resistance in Escherichia Coli ATCC25922 Under Exposure of Sub-Inhibitory Concentration of Olaquindox
antibiotics Article Development of Resistance in Escherichia coli ATCC25922 under Exposure of Sub-Inhibitory Concentration of Olaquindox Yufeng Gu 1,2, Shuge Wang 1,2, Lulu Huang 1,2, Wei Sa 1, Jun Li 1, Junhong Huang 1,2, Menghong Dai 1 and Guyue Cheng 1,2,* 1 College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China; [email protected] (Y.G.); [email protected] (S.W.); [email protected] (L.H.); [email protected] (W.S.); [email protected] (J.L.); [email protected] (J.H.); [email protected] (M.D.) 2 MOA Laboratory for Risk Assessment of Quality and Safety of Livestock and Poultry Products, Huazhong Agricultural University, Wuhan 430070, China * Correspondence: [email protected]; Tel.: +86-027-8728-7165 Received: 2 September 2020; Accepted: 8 October 2020; Published: 10 November 2020 Abstract: Quinoxaline1,4-di-N-oxides (QdNOs) are a class of important antibacterial drugs of veterinary use, of which the drug resistance mechanism has not yet been clearly explained. This study investigated the molecular mechanism of development of resistance in Escherichia coli (E. coli) under the pressure of sub-inhibitory concentration (sub-MIC) of olaquindox (OLA), a representative QdNOs drug. In vitro challenge of E. coli with 1/100 MIC to 1/2 MIC of OLA showed that the bacteria × × needed a longer time to develop resistance and could only achieve low to moderate levels of resistance as well as form weak biofilms. The transcriptomic and genomic profiles of the resistant E.