Delignification of Aspen Wood Using Hydrogen Peroxide and Peroxymonosulfate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Introduction to the Preparation and Properties of Hydiogen Peroxide
CHAPTER 1 Introduction to the Preparation and Properties of Hydiogen Peroxide 1 Introduction The following chapter will discuss the preparation of hydrogen peroxide, historically, the present day and future vistas for its in situ preparation. A brief introduction to the physical properties of hydrogen peroxide will also be made for the sake of completeness. Finally, the chapter will conclude with a practical approach to the safe handling of peroxygen species, destruction of residual peroxygens, and the toxicological and occupational health considerations required when handling hydrogen peroxide. 2 Industrial Manufacture of Hydrogen Peroxide The industrial manufacture of hydrogen peroxide can be traced back to its isolation in 18 18 by L. J. Thenard. Thenard reacted barium peroxide with nitric acid to produce a low concentration of aqueous hydrogen peroxide; the process can, however, be significantly improved by the use of hydrochloric acid. The hydrogen peroxide is formed in conjunction with barium chloride, both of which are soluble in water. The barium chloride is subsequently removed by precipitation with sulfuric acid (Figure 1.1). Hence, Thenard gave birth to the first commercial manufacture of aqueous hydrogen peroxide, although it took over sixty years before Thenard’s wet chemical process was employed in a commercial capacity.2 The industrial production of hydrogen peroxide using the above route was still operating until the middle of the 20th century. At the turn of the 19th century, approximately 10000 metric tonnes per annurn of barium peroxide were converted to about 2000 metric tonnes of hydrogen peroxide. Thenard’s process has, however, some major drawbacks which quenched the expectant explosion of its use in an aqueous form. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
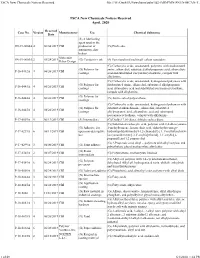
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Sulfur Dioxide in Workplace Atmospheres (Bubbler)
Withdrawn Provided For Historical Reference Only SULFUR DIOXIDE IN WORKPLACE ATMOSPHERES (BUBBLER) ♦ Method Number: ID-104 Matrix: Air OSHA PEL Sulfur Dioxide (Final Rule Limit): 2 ppm (Time Weighted Limit) 5 ppm (Short-Term Exposure Limit) Sulfur Dioxide (Transitional Limit): 5 ppm (Time Weighted Limit) Collection Device: A calibrated personal sampling pump is used to draw a known volume of air through a midget-fritted glass bubbler containing 10 to 15 mL of 0.3 N hydrogen peroxide. Recommended Air Volume: 15 to 60 L Recommended Sampling Rate: 1 L/min Analytical Procedure: Samples are directly analyzed with no sample preparation by ion chromatography as total sulfate. Detection Limit Qualitative: 0.0041 ppm (60-L air volume) Quantitative: 0.010 ppm (60-L air volume) Precision and Accuracy Validation Level: 2.5 to 10.0 ppm (60-L air volume) CVT 0.012 Bias -0.046 Overall Error ±7% Method Classification: Validated Method Chemist: Ted Wilczek, Edward Zimowski Date (Date Revised): 1981 (December, 1989) Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA. Similar products from other sources can be substituted. Branch of Inorganic Methods Development OSHA Technical Center Salt Lake City, Utah 1 of 9 Note: OSHA no longer uses or supports this method (November 2019). Withdrawn Provided For Historical Reference Only 1. Introduction This method describes the collection and analysis of airborne sulfur dioxide (SO2) using midget-fritted glass bubblers (MFGBs) in the workplace. It is applicable for both short-term (STEL) and time weighted average (TWA) exposure evaluations. -

Guidelines for the Preservation of Raw Milk by Use of the Lactoperoxidase System
GUIDELINES FOR THE PRESERVATION OF RAW MILK BY USE OF THE LACTOPEROXIDASE SYSTEM CAC/GL 13-1991 INTRODUCTION Milk is an easily perishable raw material. Contaminating bacteria may multiply rapidly and render it unsuitable for processing and/or unfit for human consumption. Bacterial growth can be retarded by refrigeration, thereby slowing down the rate of deterioration. Under certain conditions refrigeration may not be feasible due to economical and/ or technical reasons. Difficulties in applying refrigeration are specially a problem for certain areas in countries setting up or expanding their milk production. In these situations, it would be beneficial to have access to a method, other than refrigeration, for retarding bacterial growth in raw milk during collection and transportation to the dairy processing plant. In 1967 the FAO/WHO Expert Panel on Milk Quality concluded that the use of hydrogen peroxide might be an acceptable alternative in the early stages of development of an organized dairy industry, provided that certain conditions were complied with. However, this method has not achieved any general acceptance as it has several drawbacks, most important of which is the difficulty of controlling its use: it may be misused to disguise milk of basic hygienic quality produced under poor hygienic conditions. The toxicological aspects of the use of relatively high concentrations of hydrogen peroxide in milk have also been questioned. A chemical method for preserving milk would still be of great advantage in certain situations. The search for such a method has therefore continued. Interest has recently been focused on the indigenous antibacterial systems in milk to determine if these could be applied practically to preserve raw milk. -

SAFETY DATA SHEET Sodium Persulfate
SAFETY DATA SHEET Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Format: NA Version 1.06 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Sodium Persulfate CAS-No 7775-27-1 Synonyms Sodium Peroxydisulfate; Disodium Peroxydisulfate; Peroxydisulfuric acid, disodium salt; Peroxydisulfuric acid, sodium salt. Recommended use of the chemical and restrictions on use Recommended Use: Polymerization initiator; Etchant and cleaner for printed circuit boards; Hair bleaching formulations; Secondary oil recovery; Oxidizing agent for a variety of organic reactions. Restrictions on Use No uses to be advised against were identified. Manufacturer/Supplier PeroxyChem LLC 2005 Market Street Suite 3200 Philadelphia, PA 19103 Phone: +1 267/ 422-2400 (General Information) E-Mail: [email protected] Emergency telephone numbers For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) +1 303/ 389-1409 (Medical - U.S. - Call Collect) Page 1 / 10 Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Version 1.06 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2B Respiratory sensitization Category 1 Skin sensitization Category 1 Specific target organ toxicity (single exposure) Category 3 Oxidizing Solids -

19650003847.Pdf
NATIONAL BUREAU OF STANDARDS REPO RT 8595 PRELIMINARY REPORT ON A SURVEY OF THERMODYNAMIC PROPERTIES OF THE COMPOUNDS OF THE ELEMENTS CHNOPS i N65 2 CACC£S$IO NUMB£:R) CTHRU) ~ ___f£ / I- (PAGES) (CdOEI ::; ~ CIU - d -P?02c2/ :33 (NASA CR OR TMX OR AD NUMBER) (CATEQORY) Progress Report for the Period 1 August to 31 October 1964 to National Aeronautics and Space Administration GPO PRICE $ _____ OTS PRICE(S) $ 1 November 1964 Hard copy (He) of· cJV r Microfiche (MF) __....;... : .:::.j~I?J~_ <@> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARD S The ational Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. It responsibilities include development and main tena nce of the national stand· ards of measurement, and the provisions of means for making measurements consi tent with those standard ; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be nece sary, particu larl: for ~overn ment agencies; cooperation in the establi hment of standard practices for incorpora tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of device to serve special needs of the Government: assi tance to indus! r). business. and consumers in the development and acceptance of commercial tandard and simplified trade practice recommendations; administration of programs in cooperation with United tates bu iness groups and standards organizations for the development of international standard of practice; and maintenance of a clearinghouse for the collection and dis emination of scientific, tech nical. -

Organic Chemistry of Explosives
JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives Dr. Jai Prakash Agrawal CChem FRSC (UK) Former Director of Materials Defence R&D Organisation DRDO House, New Delhi, India email: [email protected] Dr. Robert Dale Hodgson Consultant Organic Chemist, Syntech Chemical Consultancy, Morecambe, Lancashire, UK Website: http://www.syntechconsultancy.co.uk email: [email protected] iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives i JWBK121-FM October 11, 2006 21:10 Char Count= 0 ii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Organic Chemistry of Explosives Dr. Jai Prakash Agrawal CChem FRSC (UK) Former Director of Materials Defence R&D Organisation DRDO House, New Delhi, India email: [email protected] Dr. Robert Dale Hodgson Consultant Organic Chemist, Syntech Chemical Consultancy, Morecambe, Lancashire, UK Website: http://www.syntechconsultancy.co.uk email: [email protected] iii JWBK121-FM October 11, 2006 21:10 Char Count= 0 Copyright C 2007 John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England Telephone (+44) 1243 779777 Email (for orders and customer service enquiries): [email protected] Visit our Home Page on www.wiley.com All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except under the terms of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London W1T 4LP, UK, without the permission in writing of the Publisher. -

Preparation of Pyridine N-Oxide Derivatives in Microreactor 2014 58 2 195
PP Periodica Polytechnica Preparation of Pyridine N-oxide Chemical Engineering Derivatives in Microreactor 58(2), pp. 195-205, 2014 DOI:10.3311/PPch.7451 Creative Commons Attribution b Attila Vörös 1, 2 * / Géza Timári 1 / Zoltán Baán 1 / Péter Mizsey 2 / Zoltán Finta 1 RESEARCH ARTICLE RECEIVED 1 APRIL 2014; ACCEPTED AFTER REVISION 12 JUNE 2014 Abstract Introduction N-oxidations of different nitrogen-containing heterocyclic Microflow technology molecules are often applied for synthetic transformations in In the recent years the importance of microreactors are con- chemistry. The industrial implementation of classical batch tinuously growing in R&D and industrial applications as well. oxidation processes are limited due to safety concerns derived This popularity originates from the unique properties of this from metal ion catalyzed decompositions. These restrictions and technology such as: very precise temperature control, outstand- other safety concern issues can be minimized or even avoided ing mixing and heat exchange due to the high surface to volume by applying glass made microreactor setups for this transfor- ratio. With an extremely low active reactor volume, ‘safety mation. The N-oxidation of different pyridine derivative, qui- concern’ chemicals and chemical processes can be handled in noline and isoquinoline is studied using two popular oxidizing safe manner under inert conditions. Based on evidence gained reagents. The further transformation of pyridine derivatives is from several examples, the scale-up of microreactors is also studied through the Polonovski rearrangement in microreactor. expected to be less problematic compared to batch processes, due to the reduced amount of critical scale-up parameters. [1] Keywords In this paper, we describe our recent results on the N-oxida- microreactor technology · N-oxide · oxidation · Polonovski tion of different pyridine derivatives, quinoline and isoquino- rearrangement · safety concern reaction line with m-CPBA in DCM or aqueous H2O2 in acetic acid using microreactor technology. -

Origins of Rotational Barriers in Hydrogen Peroxide and Hydrazine
394 J. Chem. Theory Comput. 2005, 1, 394-402 Origins of Rotational Barriers in Hydrogen Peroxide and Hydrazine Lingchun Song,† Minghong Liu,‡ Wei Wu,† Qianer Zhang,† and Yirong Mo*,†,‡ Department of Chemistry, State Key Laboratory for Physical Chemistry of Solid Surfaces, Center for Theoretical Chemistry, Xiamen UniVersity, Xiamen, Fujian 361005, P. R. China, and Department of Chemistry, Western Michigan UniVersity, Kalamazoo, Michigan 49008 Received December 21, 2004 Abstract: Compared with their isoelectronic system ethane, both hydrogen peroxide and hydrazine exhibit a double well torsional energy curve where skew conformers are favored over trans conformers and cis conformers are energy-maximum states. Clearly, the involvement of the lone oxygen and nitrogen pairs, or more specifically, the enhanced stabilizing nfσ* negative hyperconjugation effect and destabilizing repulsion among lone pairs, complicates the confor- mational analysis. In this work, the modern ab initio valence bond (VB) method is employed to quantitatively investigate the torsional energy curves of hydrogen peroxide and hydrazine in terms of hyperconjugative stabilization, steric repulsion, and structural and electronic relaxations. It is found that if the hyperconjugation effect is completely quenched, the trans conformers will be favored, while the cis conformers are the only transition state pertaining to the torsional motion in the potential energy surfaces of H2O2 and N2H4. Although usually the steric effect includes the contributions from the electronic and geometric changes, our energy decomposition analysis shows that even the steric effect favors the skew conformers, while the electronic and geometric changes stabilize the trans conformers. Thus, we conclude that both the hypercon- jugative and steric interactions lower the energy of skew conformers and eventually form low barriers from skew to trans conformers and high barriers from skew to cis conformers in both H2O2 and N2H4.