Preparing to Manufacture Hydrogen Peroxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Ammonia As a Refrigerant
1791 Tullie Circle, NE. Atlanta, Georgia 30329-2305, USA www.ashrae.org ASHRAE Position Document on Ammonia as a Refrigerant Approved by ASHRAE Board of Directors February 1, 2017 Expires February 1, 2020 ASHRAE S H A P I N G T O M O R R O W ’ S B U I L T E N V I R O N M E N T T O D A Y © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. COMMITTEE ROSTER The ASHRAE Position Document on “Ammonia as a Refrigerant” was developed by the Society’s Refrigeration Committee. Position Document Committee formed on January 8, 2016 with Dave Rule as its chair. Dave Rule, Chair Georgi Kazachki IIAR Dayton Phoenix Group Alexandria, VA, USA Dayton, OH, USA Ray Cole Richard Royal Axiom Engineers, Inc. Walmart Monterey, CA, USA Bentonville, Arkansas, USA Dan Dettmers Greg Scrivener IRC, University of Wisconsin Cold Dynamics Madison, WI, USA Meadow Lake, SK, Canada Derek Hamilton Azane Inc. San Francisco, CA, USA Other contributors: M. Kent Anderson Caleb Nelson Consultant Azane, Inc. Bethesda, MD, USA Missoula, MT, USA Cognizant Committees The chairperson of Refrigerant Committee also served as ex-officio members: Karim Amrane REF Committee AHRI Bethesda, MD, USA i © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. HISTORY of REVISION / REAFFIRMATION / WITHDRAWAL -

Introduction to the Preparation and Properties of Hydiogen Peroxide
CHAPTER 1 Introduction to the Preparation and Properties of Hydiogen Peroxide 1 Introduction The following chapter will discuss the preparation of hydrogen peroxide, historically, the present day and future vistas for its in situ preparation. A brief introduction to the physical properties of hydrogen peroxide will also be made for the sake of completeness. Finally, the chapter will conclude with a practical approach to the safe handling of peroxygen species, destruction of residual peroxygens, and the toxicological and occupational health considerations required when handling hydrogen peroxide. 2 Industrial Manufacture of Hydrogen Peroxide The industrial manufacture of hydrogen peroxide can be traced back to its isolation in 18 18 by L. J. Thenard. Thenard reacted barium peroxide with nitric acid to produce a low concentration of aqueous hydrogen peroxide; the process can, however, be significantly improved by the use of hydrochloric acid. The hydrogen peroxide is formed in conjunction with barium chloride, both of which are soluble in water. The barium chloride is subsequently removed by precipitation with sulfuric acid (Figure 1.1). Hence, Thenard gave birth to the first commercial manufacture of aqueous hydrogen peroxide, although it took over sixty years before Thenard’s wet chemical process was employed in a commercial capacity.2 The industrial production of hydrogen peroxide using the above route was still operating until the middle of the 20th century. At the turn of the 19th century, approximately 10000 metric tonnes per annurn of barium peroxide were converted to about 2000 metric tonnes of hydrogen peroxide. Thenard’s process has, however, some major drawbacks which quenched the expectant explosion of its use in an aqueous form. -

Ammonia in Drinking-Water
WHO/SDE/WSH/03.04/01 English only Ammonia in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality _______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
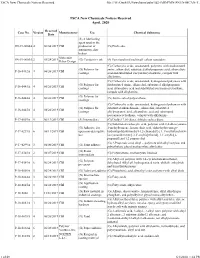
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Disinfectant Concentrations, Contact Times, and Use Settings for Products Effective Against Coronavirus SARS-Cov-2
Disinfectant Concentrations, Contact Times, and Use Settings for Products Effective against Coronavirus SARS-CoV-2 This table provides disinfectant concentrations, contact times, and use settings for EPA's List N, which covers antimicrobial products effective against SARS-CoV-2 but does not specify disinfectant concentrations. Our list will help you ensure you're using an effective disinfectant at a sufficient concentration and contact time to kill SARS-CoV-2 in appropriate settings. This table accompanies ECRI's article "Disinfectant Concentrations, Contact Times, and Use Settings for EPA's List of Products Effective against Coronavirus SARS-CoV-2, the Cause of COVID-19," available at http://ly.ecri.org/epalist. It was last updated on June 2, 2021. The Last Updated column, below, refers to the date when ECRI last reviewed EPA’s information for the product listed. EPA Reg. Primary Registered Active Disinfectant Disinfectant Disinfectant Healthcare Institutional Home Last No. Product Name Concentration Contact Time Use Use Use Updated (by weight) (min) 10190-14 Penetone XF-7117 n-Alkyl (50% C14, 40% C12, 4.34% 10 No Yes No November 10% C16) dimethyl benzyl 2020 ammonium chloride Octyl decyl dimethyl 3.25% ammonium chloride Didecyl dimethyl ammonium 1.63% chloride Dioctyl dimethyl ammonium 1.63% chloride 10324-105 Maquat 128 PD n-Alkyl (60% C14, 30% C16, 4.50% 10 Yes Yes Yes November 5% C12, 5% C18) dimethyl 2020 benzyl ammonium chloride n-Alkyl (68% C12, 32% C14) 4.50% dimethyl ethylbenzyl ammonium chloride 10324-108 Maquat 256-MN n-Alkyl -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

Ammonium Acetate
Right to Know Hazardous Substance Fact Sheet Common Name: AMMONIUM ACETATE Synonyms: None CAS Number: 631-61-8 Chemical Name: Acetic Acid, Ammonium Salt RTK Substance Number: 0085 Date: April 2002 Revision: March 2011 DOT Number: UN 9079 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Ammonium Acetate is a white, crystalline (sand-like) solid Hazard Summary with a slight vinegar-like odor. It is used in chemical analysis, Hazard Rating NJDOH NFPA textile dyeing, and preserving meats. HEALTH 2 - FLAMMABILITY 1 - REACTIVITY 0 - POISONOUS GASES ARE PRODUCED IN FIRE Reasons for Citation f Ammonium Acetate is on the Right to Know Hazardous Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe Substance List because it is cited by DOT and IRIS. f Ammonium Acetate can affect you when inhaled. f Contact can irritate and burn the skin and eyes. f Inhaling Ammonium Acetate can irritate the nose, throat and lungs causing coughing, wheezing and/or shortness of breath. SEE GLOSSARY ON PAGE 5. FIRST AID Eye Contact Workplace Exposure Limits f Immediately flush with large amounts of water for at least 30 No occupational exposure limits have been established for minutes, lifting upper and lower lids. Remove contact Ammonium Acetate. However, it may pose a health risk. lenses, if worn, while flushing. Seek medical attention. Always follow safe work practices. Skin Contact f Quickly remove contaminated clothing. Immediately wash contaminated skin with large amounts of water. Inhalation f Remove the person from exposure. f Begin rescue breathing (using universal precautions) if breathing has stopped and CPR if heart action has stopped. -

Sulfur Dioxide in Workplace Atmospheres (Bubbler)
Withdrawn Provided For Historical Reference Only SULFUR DIOXIDE IN WORKPLACE ATMOSPHERES (BUBBLER) ♦ Method Number: ID-104 Matrix: Air OSHA PEL Sulfur Dioxide (Final Rule Limit): 2 ppm (Time Weighted Limit) 5 ppm (Short-Term Exposure Limit) Sulfur Dioxide (Transitional Limit): 5 ppm (Time Weighted Limit) Collection Device: A calibrated personal sampling pump is used to draw a known volume of air through a midget-fritted glass bubbler containing 10 to 15 mL of 0.3 N hydrogen peroxide. Recommended Air Volume: 15 to 60 L Recommended Sampling Rate: 1 L/min Analytical Procedure: Samples are directly analyzed with no sample preparation by ion chromatography as total sulfate. Detection Limit Qualitative: 0.0041 ppm (60-L air volume) Quantitative: 0.010 ppm (60-L air volume) Precision and Accuracy Validation Level: 2.5 to 10.0 ppm (60-L air volume) CVT 0.012 Bias -0.046 Overall Error ±7% Method Classification: Validated Method Chemist: Ted Wilczek, Edward Zimowski Date (Date Revised): 1981 (December, 1989) Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA. Similar products from other sources can be substituted. Branch of Inorganic Methods Development OSHA Technical Center Salt Lake City, Utah 1 of 9 Note: OSHA no longer uses or supports this method (November 2019). Withdrawn Provided For Historical Reference Only 1. Introduction This method describes the collection and analysis of airborne sulfur dioxide (SO2) using midget-fritted glass bubblers (MFGBs) in the workplace. It is applicable for both short-term (STEL) and time weighted average (TWA) exposure evaluations. -

Guidelines for the Preservation of Raw Milk by Use of the Lactoperoxidase System
GUIDELINES FOR THE PRESERVATION OF RAW MILK BY USE OF THE LACTOPEROXIDASE SYSTEM CAC/GL 13-1991 INTRODUCTION Milk is an easily perishable raw material. Contaminating bacteria may multiply rapidly and render it unsuitable for processing and/or unfit for human consumption. Bacterial growth can be retarded by refrigeration, thereby slowing down the rate of deterioration. Under certain conditions refrigeration may not be feasible due to economical and/ or technical reasons. Difficulties in applying refrigeration are specially a problem for certain areas in countries setting up or expanding their milk production. In these situations, it would be beneficial to have access to a method, other than refrigeration, for retarding bacterial growth in raw milk during collection and transportation to the dairy processing plant. In 1967 the FAO/WHO Expert Panel on Milk Quality concluded that the use of hydrogen peroxide might be an acceptable alternative in the early stages of development of an organized dairy industry, provided that certain conditions were complied with. However, this method has not achieved any general acceptance as it has several drawbacks, most important of which is the difficulty of controlling its use: it may be misused to disguise milk of basic hygienic quality produced under poor hygienic conditions. The toxicological aspects of the use of relatively high concentrations of hydrogen peroxide in milk have also been questioned. A chemical method for preserving milk would still be of great advantage in certain situations. The search for such a method has therefore continued. Interest has recently been focused on the indigenous antibacterial systems in milk to determine if these could be applied practically to preserve raw milk. -

Ammonium Nitrate
8.3 Ammonium Nitrate 8.3.1 General1-3 Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3). In 1991, there were 58 U. S. ammonium nitrate plants located in 22 states producing about 8.2 million megagrams (Mg) (9 million tons) of ammonium nitrate. Approximately 15 to 20 percent of this amount was used for explosives and the balance for fertilizer. Ammonium nitrate is marketed in several forms, depending upon its use. Liquid ammonium nitrate may be sold as a fertilizer, generally in combination with urea. Liquid ammonium nitrate may be concentrated to form an ammonium nitrate "melt" for use in solids formation processes. Solid ammonium nitrate may be produced in the form of prills, grains, granules, or crystals. Prills can be produced in either high or low density form, depending on the concentration of the melt. High density prills, granules, and crystals are used as fertilizer, grains are used solely in explosives, and low density prills can be used as either. 8.3.2 Process Description1,2 The manufacture of ammonium nitrate involves several major unit operations including solution formation and concentration; solids formation, finishing, screening, and coating; and product bagging and/or bulk shipping. In some cases, solutions may be blended for marketing as liquid fertilizers. These operations are shown schematically in Figure 8.3-1. The number of operating steps employed depends on the end product desired. For example, plants producing ammonium nitrate solutions alone use only the solution formation, solution blending, and bulk shipping operations. Plants producing a solid ammonium nitrate product may employ all of the operations.